Page 1184 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1184
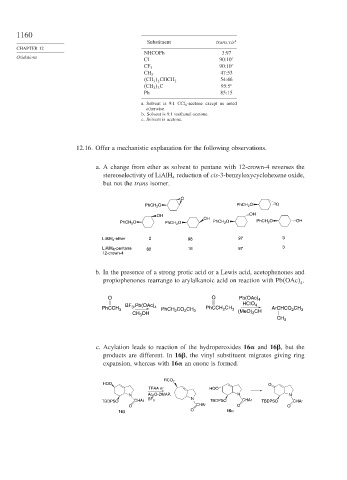
1160
Substituent trans:cis a
CHAPTER 12
NHCOPh 3:97
Oxidations c
Cl 90:10
90:10 c
CF 3
47:53
CH 3
54:46
CH 3 2 CHCH 2
CH 3 3 C 95:5 c
Ph 85:15
a. Solvent is 9:1 CCl 4 -acetone except as noted
otherwise.
b. Solvent is 9:1 methanol-acetone.
c. Solvent is acetone.
12.16. Offer a mechanistic explanation for the following observations.
a. A change from ether as solvent to pentane with 12-crown-4 reverses the
stereoselectivity of LiAlH reduction of cis-3-benzyloxycyclohexene oxide,
4
but not the trans isomer.
O
PhCH 2 O PhCH 2 O O
OH OH
OH
PhCH 2 O PhCH 2 O PhCH 2 O PhCH 2 O OH
LiAlH 4 -ether 2 98 97 3
LiAlH 4 -pentane 82 18 97 3
12-crown-4
b. In the presence of a strong protic acid or a Lewis acid, acetophenones and
propiophenones rearrange to arylalkanoic acid on reaction with Pb OAc .
4
O O Pb(OAc) 4
BF 3 ,Pb(OAc) 4 HClO 4
PhCCH 3 PhCH 2 CO 2 CH 3 PhCCH 2 CH 3 (MeO) 3 CH ArCHCO 2 CH 3
CH 3 OH
CH 3
c. Acylation leads to reaction of the hydroperoxides 16 and 16 , but the
products are different. In 16 , the vinyl substituent migrates giving ring
expansion, whereas with 16 an enone is formed.
RCO 2
HOO O
TFAA or HOO
N Ac 2 O-DMAP, N N
TBDPSO CHAr BF 3 N TBDPSO CHAr TBDPSO CHAr
O CHAr O O
16β O 16α