Page 1183 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1183
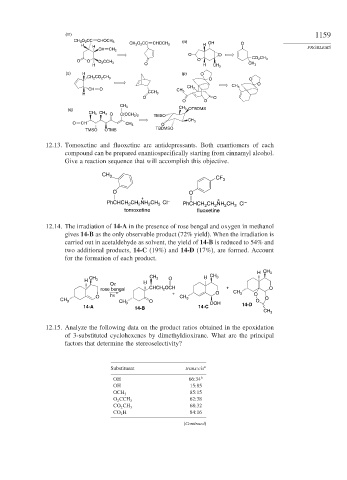
(m) 1159
CH 3 O 2 CC CHOCH 3 (n)
H H CH 3 O 2 CC CHOCH 3 H OH O
CH CH 2 PROBLEMS
O O
O O O 2 CCH 3 O CO 2 CH 3
H O H CH 3 CH 3
(o) H (p) O
CH 2 CO 2 CH 3
O O
O
CH O CH 3 CH 3 CH 3
H CCH 3
O O
O O
CH 3
(q) CH 3 OTBDMS
CH 3 CH 3 O
C(OCH 3 ) 2 TMSO
CH 3
O CH CH 3 O
TBDMSO
TMSO OTMS
12.13. Tomoxetine and fluoxetine are antidepressants. Both enantiomers of each
compound can be prepared enantiospecifically starting from cinnamyl alcohol.
Give a reaction sequence that will accomplish this objective.
CH 3 CF 3
O O
+ +
PhCHCH CH NH CH Cl – PhCHCH CH NH CH Cl –
3
2
2
2
2
3
2
2
tomoxetine fluoxetine
12.14. The irradiation of 14-A in the presence of rose bengal and oxygen in methanol
gives 14-B as the only observable product (72% yield). When the irradiation is
carried out in acetaldehyde as solvent, the yield of 14-B is reduced to 54% and
two additional products, 14-C (19%) and 14-D (17%), are formed. Account
for the formation of each product.
H CH 3
CH 3 O H CH 3
H CH 3 H
O2
rose bengal CHCH 2 OCH + O
hν + O CH 3 O
O CH 3 O
CH 3 O O
CH 3 OOH 14-D
14-A 14-B 14-C
CH 3
12.15. Analyze the following data on the product ratios obtained in the epoxidation
of 3-substituted cyclohexenes by dimethyldioxirane. What are the principal
factors that determine the stereoselectivity?
Substituent trans:cis a
OH 66:34 b
OH 15:85
85:15
OCH 3
62:38
O 2 CCH 3
68:32
CO 2 CH 3
CO 2 H 84:16
(Continued)