Page 1191 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1191
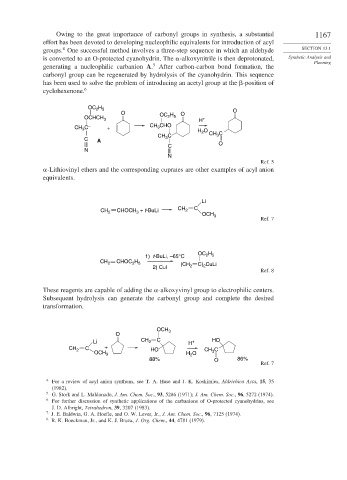
Owing to the great importance of carbonyl groups in synthesis, a substantial 1167
effort has been devoted to developing nucleophilic equivalents for introduction of acyl
4
groups. One successful method involves a three-step sequence in which an aldehyde SECTION 13.1
is converted to an O-protected cyanohydrin. The -alkoxynitrile is then deprotonated, Synthetic Analysis and
Planning
5
generating a nucleophilic carbanion A. After carbon-carbon bond formation, the
carbonyl group can be regenerated by hydrolysis of the cyanohydrin. This sequence
has been used to solve the problem of introducing an acetyl group at the -position of
cyclohexenone. 6
OC H
2 5
O H O O
OCHCH 3 OC 2 5 H +
CH C – + CH 3 CHO H O
3
CH C 2 CH 3 C
C A 3
C O
N
N
Ref. 5
-Lithiovinyl ethers and the corresponding cuprates are other examples of acyl anion
equivalents.
Li
CH C
CH 2 CHOCH + t-BuLi 2
3
OCH 3
Ref. 7
OC H
1) t-BuLi, –65°C 2 5
CH 2 CHOC H (CH C) CuLi
2 5
2) CuI 2 2
Ref. 8
These reagents are capable of adding the -alkoxyvinyl group to electrophilic centers.
Subsequent hydrolysis can generate the carbonyl group and complete the desired
transformation.
OCH 3
O
Li CH 2 C H + HO
CH 2 C + HO C
OCH 3 H O CH 3
2
88% O 86%
Ref. 7
4 For a review of acyl anion synthons, see T. A. Hase and J. K. Koskimies, Aldrichica Acta, 15,35
(1982).
5 G. Stork and L. Maldonado, J. Am. Chem. Soc., 93, 5286 (1971); J. Am. Chem. Soc., 96, 5272 (1974).
6
For further discussion of synthetic applications of the carbanions of O-protected cyanohydrins, see
J. D. Albright, Tetrahedron, 39, 3207 (1983).
7 J. E. Baldwin, G. A. Hoefle, and O. W. Lever, Jr., J. Am. Chem. Soc., 96, 7125 (1974).
8
R. K. Boeckman, Jr., and K. J. Bruza, J. Org. Chem., 44, 4781 (1979).