Page 1195 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1195
O 1171
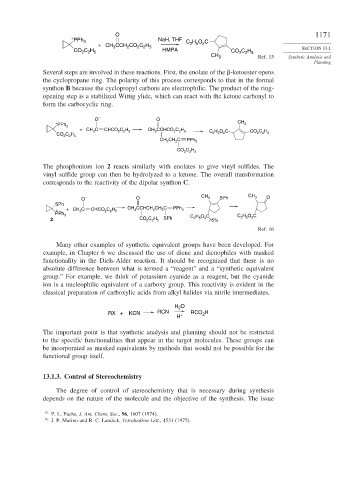
+
PPh 3 NaH, THF H O C
+ CH CCH CO C H C 2 5 2
2 2 5
2
3
CO C H HMPA CO C H SECTION 13.1
2 2 5
2 2 5
CH 3 Ref. 15 Synthetic Analysis and
Planning
Several steps are involved in these reactions. First, the enolate of the -ketoester opens
the cyclopropane ring. The polarity of this process corresponds to that in the formal
synthon B because the cyclopropyl carbons are electrophilic. The product of the ring-
opening step is a stabilized Wittig ylide, which can react with the ketone carbonyl to
form the carbocyclic ring.
O – O
+ CH
PPh 3 3
+ CH C CHCO 2 C H CH CCHCO C H C H O C
CO C H 3 2 5 3 2 2 5 2 5 2 CO 2 C 2 H 5
2 2 5
CH C PPh
CH 2 2 3
CO C H 5
2
2
The phosphonium ion 2 reacts similarly with enolates to give vinyl sulfides. The
vinyl sulfide group can then be hydrolyzed to a ketone. The overall transformation
corresponds to the reactivity of the dipolar synthon C.
O – O CH 3 SPh CH 3 O
SPh
3
2
+ + CH C CHCO C H 5 CH CCHCH CH C PPh 3
2
2
2
3
PPh 3 C H O C C H O C
2 CO 2 C H 5 SPh 2 5 2 75% 2 5 2
2
Ref. 16
Many other examples of synthetic equivalent groups have been developed. For
example, in Chapter 6 we discussed the use of diene and dienophiles with masked
functionality in the Diels-Alder reaction. It should be recognized that there is no
absolute difference between what is termed a “reagent” and a “synthetic equivalent
group.” For example, we think of potassium cyanide as a reagent, but the cyanide
ion is a nucleophilic equivalent of a carboxy group. This reactivity is evident in the
classical preparation of carboxylic acids from alkyl halides via nitrile intermediates.
H O
2
RX + KCN RCN RCO H
2
H +
The important point is that synthetic analysis and planning should not be restricted
to the specific functionalities that appear in the target molecules. These groups can
be incorporated as masked equivalents by methods that would not be possible for the
functional group itself.
13.1.3. Control of Stereochemistry
The degree of control of stereochemistry that is necessary during synthesis
depends on the nature of the molecule and the objective of the synthesis. The issue
15 P. L. Fuchs, J. Am. Chem. Soc., 96, 1607 (1974).
16
J. P. Marino and R. C. Landick, Tetrahedron Lett., 4531 (1975).