Page 1194 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1194
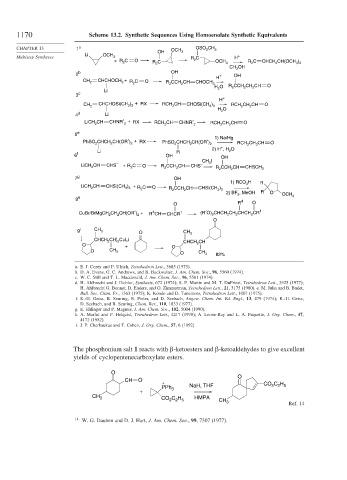
1170 Scheme 13.2. Synthetic Sequences Using Homoenolate Synthetic Equivalents
CHAPTER 13 1 a OCH OSO 2 CH 3
Li OCH OH 3
Multistep Syntheses 3 R C H +
+R C O R C 2 OCH C CHCH CH(OCH )
2 3 R 2 2 3 2
2
CH OH
3
b OH
2 + OH
H
CH 2 CHCHOCH + R C O R 2 CCH CH CHOCH 3
3
2
2
H O R CCH CH CH O
2
2
2
Li 2
3 c
H +
) +
CH 2 CHCHOSi(CH 3 3 RX RCH CH CHOSi(CH ) RCH CH CH O
2
2
3 3
2
H O
2
4 d Li
CH + RX CHNR′
LiCH 2 CHNR′ 2 RCH CH 2 RCH CH CH O
2
2
2
5 e
1) Na/Hg
CHCH CH(OR′) + RX PhSO CHCH CH(OR′)
PhSO 2 2 2 2 2 2 RCH CH CH O
2
2
+
2) H , H O
Li R 2
6 f OH OH
CH 3 I
LiCH CH CHS – +R 2 C O R CCH CH CHS –
2 2 2 R CCH CH CHSCH 3
2 2
7 g OH
1) RCO H R
LiCH CH ) C O 3
2 CHSi(CH 3 3 + R 2 R CCH CH CHSi(CH )
2 2 3 3
2) BF , MeOH R O
3 OCH 3
8 h 4
O R O
CH CH(OR′) + 4 1 (R′O) CHCH CH CHCH CR 1
CuBr/BrMgCH 2 2 2 R CH CHCR 2 2 2 2
O
9 i CH 3 O CH 3
[ CHCH CH] CuLi CHCH CH
2
2
O + O 2
O CH CH
3
O 3 82%
a. E. J. Corey and P. Ulrich, Tetrahedron Lett., 3685 (1975).
b. D. A. Evans, G. C. Andrews, and B. Buckwalter, J. Am. Chem. Soc., 96, 5560 (1974).
c. W. C. Still and T. L. Macdonald, J. Am. Chem. Soc., 96, 5561 (1974).
d. H. Ahlbrecht and J. Eichler, Synthesis, 672 (1974); S. F. Martin and M. T. DuPriest, Tetrahedron Lett., 3925 (1977);
H. Ahlbrecht G. Bonnet, D. Enders, and G. Zimmerman, Tetrahedron Lett., 21, 3175 (1980). e. M. Julia and B. Badet,
Bull. Soc. Chim. Fr., 1363 (1975); K. Kondo and D. Tunemoto, Tetrahedron Lett., 1007 (1975).
f. K.-H. Geiss, B. Seuring, R. Pieter, and D. Seebach, Angew. Chem. Int. Ed. Engl., 13, 479 (1974); K.-H. Geiss,
D. Seebach, and B. Seuring, Chem. Ber., 110, 1833 (1977).
g. E. Ehlinger and P. Magnus, J. Am. Chem. Soc., 102, 5004 (1990).
h. A. Marfat and P. Helquist, Tetrahedron Lett., 4217 (1978); A. Leone-Bay and L. A. Paquette, J. Org. Chem., 47,
4172 (1982).
i. J. P. Cherkaukas and T. Cohen, J. Org. Chem., 57, 6 (1992).
The phosphonium salt 1 reacts with -ketoesters and -ketoaldehydes to give excellent
yields of cyclopentenecarboxylate esters.
O
O
CH O
+ CO C H
PPh 3 NaH, THF 2 2 5
+
CO C H
CH 3 HMPA
2 2 5
CH 3
Ref. 14
14
W. G. Dauben and D. J. Hart, J. Am. Chem. Soc., 99, 7307 (1977).