Page 1202 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1202
1178
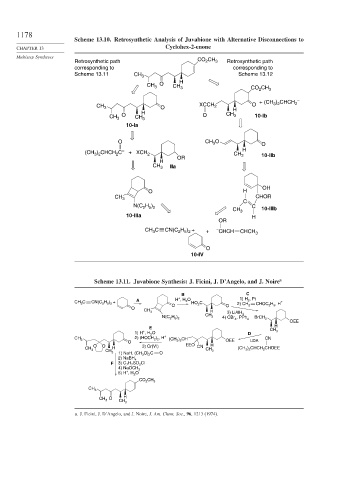
Scheme 13.10. Retrosynthetic Analysis of Juvabione with Alternative Disconnections to
CHAPTER 13 Cyclohex-2-enone
Multistep Syntheses
Retrosynthetic path CO CH 3 Retrosynthetic path
2
corresponding to corresponding to
Scheme 13.11 CH 3 Scheme 13.12
H
CH 3 O CH 3 CO CH 3
2
–
) CHCH
CH 3 O XCCH 2 H O + (CH 3 2 2
CH 3 O CH H 3 O CH 3 10-Ib
10-Ia
O CH O O
3
–
(CH ) CHCH C + XCH 2 CH 3 H
2
3 2
OR 10-IIb
H
CH 3 IIa
OH
O H
CH 3 CHOR
C
N(C H ) C
2 5 2
CH 3 10-IIIb
10-IIIa H
OR
–
CH C CN(C H ) + + CHCH CHCH 3
3
2 5 2
O
10-IV
Scheme 13.11. Juvabione Synthesis: J. Ficini, J. D’Angelo, and J. Noire a
B C
+
CH 3 C CN(C 2 H 5 ) 2 + A H , H 2 O HO 2 C 1) H 2 , Pt CHOC 2 H 5 , H +
O O 2) CH 2
O
CH 3 H
3) LiAlH 4
CH 3
N(C 2 H 5 ) 2
4) CBr 4 , PPh 4 BrCH 2
OEE
H
E
+ CH 3
1) H , H O D
2
2) (HOCH 2 ) 2 , H +
CH 3 (CH 3 ) 2 CH OEE LDA CN
O
O O 3) Cr(VI) EEO CN H
CH 3 H (CH 3 ) 2 CHCH 2 CHOEE
CH 3
1) NaH, (CH 3 O) 2 C O
CH 3
2) NaBH 4
F 3) C 7 H 7 SO 2 Cl
4) NaOCH 3
+
5) H , H 2 O
CO 2 CH 3
CH 3
O H
CH 3
CH 3
a. J. Ficini, J. D’Angelo, and J. Noire, J. Am. Chem. Soc., 96, 1213 (1974).