Page 1204 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1204
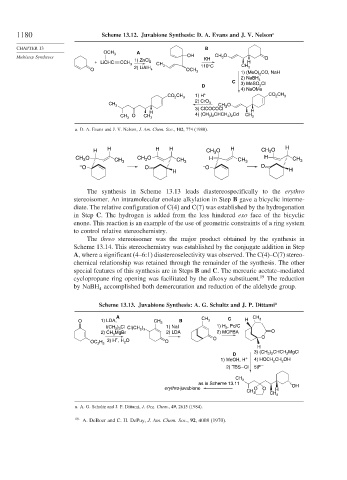
1180 Scheme 13.12. Juvabione Synthesis: D. A. Evans and J. V. Nelson a
CHAPTER 13 B
OCH 3 A
Multistep Syntheses OH KH CH O O
3
+ LiCHC CCH 3 1) ZnCl 2 CH H
O 2) LiAlH 4 3 OCH 3 110°C CH 3
1) (MeO) 2 CO, NaH
2) NaBH 4
C
D 3) MeSO 2 Cl
4) NaOMe
CO CH 1) H + CO 2 CH 3
2 3
2) CrO
CH 3 3 CH 3 O
3) ClCOCOCl
H H
) CHCH ) Cd
CH 3 O CH 3 4) (CH 3 2 2 2 CH 3
a. D. A. Evans and J. V. Nelson, J. Am. Chem. Soc., 102, 774 (1980).
H H H H CH O H CH 3 O H
3
3
CH 3 O CH 3 CH O CH 3 H CH 3 H CH 3
– O O – O O
H H
The synthesis in Scheme 13.13 leads diastereospecifically to the erythro
stereoisomer. An intramolecular enolate alkylation in Step B gave a bicyclic interme-
diate. The relative configuration of C(4) and C(7) was established by the hydrogenation
in Step C. The hydrogen is added from the less hindered exo face of the bicyclic
enone. This reaction is an example of the use of geometric constraints of a ring system
to control relative stereochemistry.
The threo stereoisomer was the major product obtained by the synthesis in
Scheme 13.14. This stereochemistry was established by the conjugate addition in Step
A, where a significant (4–6:1) diastereoselectivity was observed. The C(4)–C(7) stereo-
chemical relationship was retained through the remainder of the synthesis. The other
special features of this synthesis are in Steps B and C. The mercuric acetate–mediated
cyclopropane ring opening was facilitated by the alkoxy substituent. 19 The reduction
by NaBH accomplished both demercuration and reduction of the aldehyde group.
4
Scheme 13.13. Juvabione Synthesis: A. G. Schultz and J. P. Dittami a
A CH CH
O 1) LDA, CH 3 B 3 C H 3
I(CH ) Cl Cl(CH 2 3 1) NaI 1) H 2 , Pd/C
)
2 3
2) CH MgBr 2) LDA 2) MCPBA O
3
+
OC H 5 3) H , H O O O O
2
2
H
3) (CH ) CHCH MgCl
D 3 2 2
+
1) MeOH, H 4) HOCH 2 CH OH
2
–
2) TBS – Cl 5)F
CH 3
as in Scheme 13.11 OH
erythro-juvabione O O H
CH 3 CH 3
a. A. G. Schultz and J. P. Dittami, J. Org. Chem., 49, 2615 (1984).
19
A. DeBoer and C. H. DePuy, J. Am. Chem. Soc., 92, 4008 (1970).