Page 1209 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1209
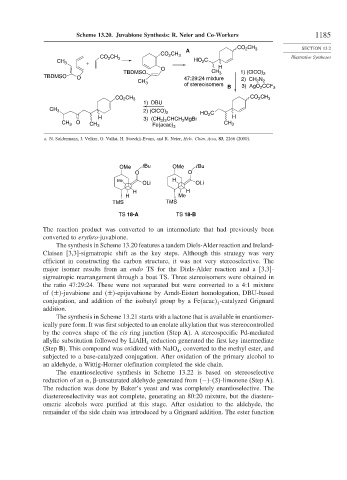
Scheme 13.20. Juvabione Synthesis: R. Neier and Co-Workers 1185
CO 2 CH 3 SECTION 13.2
A
CO CH
CO CH 3 2 3 Illustrative Syntheses
2
2
CH 3 HO C
+
O H
TBDMSO CH 3 1) (ClCO) 2
TBDMSO O 47:29:24 mixture 2) CH N
CH 3 2 2
of stereoisomers
B 3) AgO 2 CCF 3
CO CH
CO 2 CH 3 2 3
1) DBU
CH 3 2) (ClCO) 2 HO C
H ) CHCH MgBr 2 H
CH 3 O CH 3 3) (CH 3 2 2 CH 3
Fe(acac) 3
a. N. Soldermann, J. Velker, O. Vallat, H. Stoeckli-Evans, and R. Neier, Helv. Chim. Acta, 83, 2266 (2000).
OMe t Bu OMe t Bu
O O
Me H
OLi OLi
H H
H Me
TMS TMS
TS 18-A TS 18-B
The reaction product was converted to an intermediate that had previously been
converted to erythro-juvabione.
The synthesis in Scheme 13.20 features a tandem Diels-Alder reaction and Ireland-
Claisen [3,3]-sigmatropic shift as the key steps. Although this strategy was very
efficient in constructing the carbon structure, it was not very stereoselective. The
major isomer results from an endo TS for the Diels-Alder reaction and a [3,3]-
sigmatropic rearrangement through a boat TS. Three stereoisomers were obtained in
the ratio 47:29:24. These were not separated but were converted to a 4:1 mixture
of ± -juvabione and ± -epijuvabione by Arndt-Eistert homologation, DBU-based
conjugation, and addition of the isobutyl group by a Fe acac -catalyzed Grignard
3
addition.
The synthesis in Scheme 13.21 starts with a lactone that is available in enantiomer-
ically pure form. It was first subjected to an enolate alkylation that was stereocontrolled
by the convex shape of the cis ring junction (Step A). A stereospecific Pd-mediated
allylic substitution followed by LiAlH reduction generated the first key intermediate
4
(Step B). This compound was oxidized with NaIO , converted to the methyl ester, and
4
subjected to a base-catalyzed conjugation. After oxidation of the primary alcohol to
an aldehyde, a Wittig-Horner olefination completed the side chain.
The enantioselective synthesis in Scheme 13.22 is based on stereoselective
reduction of an -unsaturated aldehyde generated from − - S -limonene (Step A).
The reduction was done by Baker’s yeast and was completely enantioselective. The
diastereoselectivity was not complete, generating an 80:20 mixture, but the diastere-
omeric alcohols were purified at this stage. After oxidation to the aldehyde, the
remainder of the side chain was introduced by a Grignard addition. The ester function