Page 1212 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1212
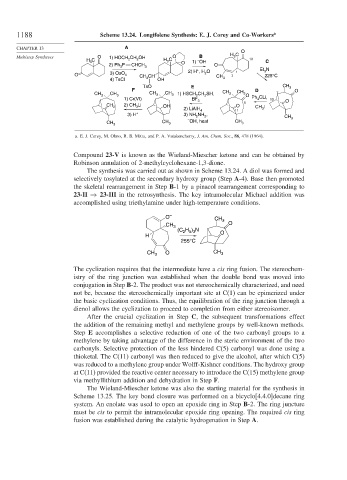
1188 Scheme 13.24. Longifolene Synthesis: E. J. Corey and Co-Workers a
CHAPTER 13 A
O
H C
Multistep Syntheses O 1) HOCH CH OH O B 3
–
H 3 C 2 2 H C O 1) OH 10 C
3
2) Ph P CHCH 3 O
3
+
2) H , H O 1 Et N
3
O 3) OsO 4 CH CH 2 CH 7 225°C
4) TsCl 3 OH 3
TsO E CH 3
F D O
CH 3 CH 3 CH 3 CH 3 1) HSCH CH SH, CH 3 CH 3 O 7
2
2
1) Cr(VI) BF 3 Ph CLi, 10
3
5 O
CH 2) CH Li OH O CH I
2
3
2) LiAlH 3
4 11
3) H + 3) NH NH ,
2 2 CH 3
– OH, heat CH
CH 3 CH 3 3
a. E. J. Corey, M. Ohno, R. B. Mitra, and P. A. Vatakencherry, J. Am. Chem. Soc., 86, 478 (1964).
Compound 23-V is known as the Wieland-Miescher ketone and can be obtained by
Robinson annulation of 2-methylcyclohexane-1,3-dione.
The synthesis was carried out as shown in Scheme 13.24. A diol was formed and
selectively tosylated at the secondary hydroxy group (Step A-4). Base then promoted
the skeletal rearrangement in Step B-1 by a pinacol rearrangement corresponding to
23-II ⇒ 23-III in the retrosynthesis. The key intramolecular Michael addition was
accomplished using triethylamine under high-temperature conditions.
O –
CH 3
CH 3 O
(C H ) N
2 5 3
H O
255°C
CH 3 O CH 3
The cyclization requires that the intermediate have a cis ring fusion. The stereochem-
istry of the ring junction was established when the double bond was moved into
conjugation in Step B-2. The product was not stereochemically characterized, and need
not be, because the stereochemically important site at C(1) can be epimerized under
the basic cyclization conditions. Thus, the equilibration of the ring junction through a
dienol allows the cyclization to proceed to completion from either stereoisomer.
After the crucial cyclization in Step C, the subsequent transformations effect
the addition of the remaining methyl and methylene groups by well-known methods.
Step E accomplishes a selective reduction of one of the two carbonyl groups to a
methylene by taking advantage of the difference in the steric environment of the two
carbonyls. Selective protection of the less hindered C(5) carbonyl was done using a
thioketal. The C(11) carbonyl was then reduced to give the alcohol, after which C(5)
was reduced to a methylene group under Wolff-Kishner conditions. The hydroxy group
at C(11) provided the reactive center necessary to introduce the C(15) methylene group
via methyllithium addition and dehydration in Step F.
The Wieland-Miescher ketone was also the starting material for the synthesis in
Scheme 13.25. The key bond closure was performed on a bicyclo[4.4.0]decane ring
system. An enolate was used to open an epoxide ring in Step B-2. The ring juncture
must be cis to permit the intramolecular epoxide ring opening. The required cis ring
fusion was established during the catalytic hydrogenation in Step A.