Page 1213 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1213
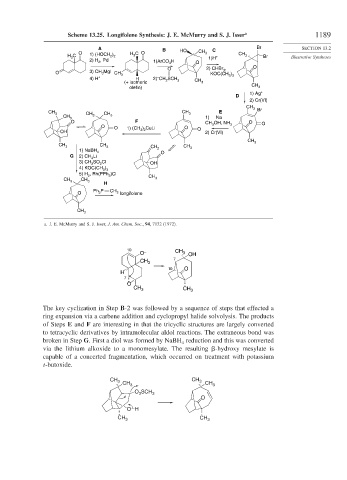
Scheme 13.25. Longifolene Synthesis: J. E. McMurry and S. J. Isser a 1189
A B HO C Br SECTION 13.2
O C O CH 3 CH
H C 1) (HOCH ) H 3 1)H + 3 Br Illustrative Syntheses
2 2
3
2) H , Pd 1)ArCO 3 H O
2
O 2) CHBr , O
3
O 3) CH MgI CH 3 KOC(CH 3 3
3
)
–
4) H + H 2) CH SCH CH
(+ isomeric 2 3 3
olefin) CH 3
1) Ag +
D
2) Cr(VI)
CH 3 Br
CH CH E
3 CH CH 3
CH 3 3 3 1) Na
O F OH, NH O
CH 3 O
O O ) CuLi O 3
OH 1) (CH 3 2 O 2) Cr(VI)
CH
CH CH CH 3
3 3 CH
2 3
1) NaBH 4 O
G 2) CH Li
3
3) CH SO Cl OH
2
3
)
4) KOC(CH 3 3
5) H , Rh(PPh )Cl
3
2
CH CH CH 3
3 3
H
P CH 2 longifolene
O Ph 3
CH 3
a. J. E. McMurry and S. J. Isser, J. Am. Chem. Soc., 94, 7132 (1972).
10 CH
O – 3 OH
CH 3 7
10 O
H
7
O
CH 3 CH 3
The key cyclization in Step B-2 was followed by a sequence of steps that effected a
ring expansion via a carbene addition and cyclopropyl halide solvolysis. The products
of Steps E and F are interesting in that the tricyclic structures are largely converted
to tetracyclic derivatives by intramolecular aldol reactions. The extraneous bond was
broken in Step G. First a diol was formed by NaBH reduction and this was converted
4
via the lithium alkoxide to a monomesylate. The resulting -hydroxy mesylate is
capable of a concerted fragmentation, which occurred on treatment with potassium
t-butoxide.
CH CH
3
CH 3 3 CH 3
O 3 SCH 3
O
O H
CH 3 CH 3