Page 1218 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1218
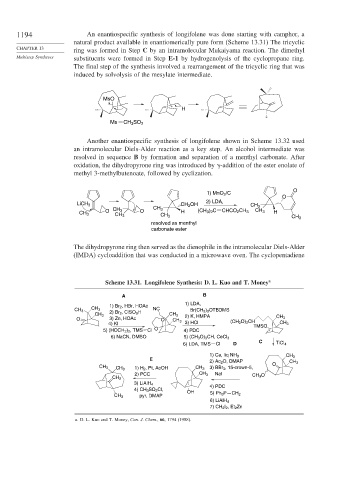
1194 An enantiospecific synthesis of longifolene was done starting with camphor, a
natural product available in enantiomerically pure form (Scheme 13.31) The tricyclic
CHAPTER 13
ring was formed in Step C by an intramolecular Mukaiyama reaction. The dimethyl
Multistep Syntheses substituents were formed in Step E-1 by hydrogenolysis of the cyclopropane ring.
The final step of the synthesis involved a rearrangement of the tricyclic ring that was
induced by solvolysis of the mesylate intermediate.
MsO
+
H
Ms CH 3 SO 2
Another enantiospecific synthesis of longifolene shown in Scheme 13.32 used
an intramolecular Diels-Alder reaction as a key step. An alcohol intermediate was
resolved in sequence B by formation and separation of a menthyl carbonate. After
oxidation, the dihydropyrone ring was introduced by -addition of the ester enolate of
methyl 3-methylbutenoate, followed by cyclization.
O
1) MnO 2 /C
O
– 2) LDA,
LiCH 3 CH 2 OH CH 3
CH 3
O CH 3 O H (CH 3 ) 2 C CHCO 2 CH 3 CH 3 H
CH 3
CH 3 CH 3
CH 3
resolved as menthyl
carbonate ester
The dihydropyrone ring then served as the dienophile in the intramolecular Diels-Alder
(IMDA) cycloaddition that was conducted in a microwave oven. The cyclopentadiene
Scheme 13.31. Longifolene Synthesis: D. L. Kuo and T. Money a
A B
1) LDA,
1) Br 2 , HBr, HOAc
CH 3 NC Br(CH 2 ) 3 OTBDMS
CH 3 2) Br 2 , ClSO 3 H
CH 3 CH 3 2) K, HMPA
O 3) Zn, HOAc O CH 3 (CH 3 O) 2 CH CH 3
4) KI 3) HCl TMSO CH 3
5) (HOCH 2 ) 2 , TMS Cl O 4) PDC
6) NaCN, DMSO 5) (CH 3 O) 3 CH, CeCl 3
C
6) LDA, TMS Cl D TiCl 4
1) Ca, liq NH 3 CH 3
E
2) Ac 2 O, DMAP CH 3
CH 3 1) H 2 , Pt, AcOH CH 3 3) BBr 3 , 15-crown-5, O
CH 3
2) PCC CH 3 NaI CH 3 O
CH 2
3) LiAlH 4
4) PDC
4) CH 3 SO 2 Cl,
OH 5) Ph 3 P
pyr, DMAP CH 2
CH 3
6) LiAlH 4
7) CH 2 I 2 , Et 2 Zn
a. D. L. Kuo and T. Money, Can. J. Chem., 66, 1794 (1988).