Page 1222 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1222
1198 H CH 2 OTBDMS CH OTBDMS
2
C CH 3 H CH
CHAPTER 13 CH 3 O C 3
CH 3 O
Multistep Syntheses
CH 3 O – CH 3 O
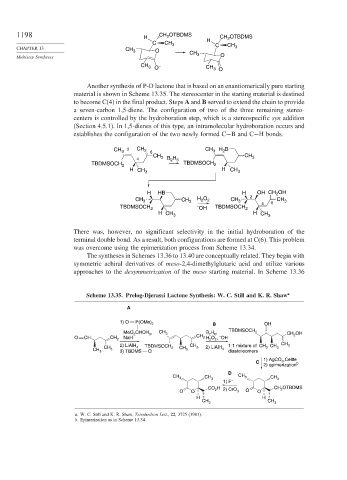
Another synthesis of P-D lactone that is based on an enantiomerically pure starting
material is shown in Scheme 13.35. The stereocenter in the starting material is destined
to become C(4) in the final product. Steps A and B served to extend the chain to provide
a seven-carbon 1,5-diene. The configuration of two of the three remaining stereo-
centers is controlled by the hydroboration step, which is a stereospecific syn addition
(Section 4.5.1). In 1,5-dienes of this type, an intramolecular hydroboration occurs and
establishes the configuration of the two newly formed C−B and C−H bonds.
CH 3 2 CH 2 6 CH 3 H 2 B
CH 3 CH 3
4 B H
2 6
TBDMSOCH 2 TBDMSOCH 2
H CH H CH
3 3
H HB H OH CH OH
2
CH 3 CH 3 H O 2 CH 3 2 6 CH 3
2
TBDMSOCH 2 – OH TBDMSOCH 2 4
H CH 3 H CH 3
There was, however, no significant selectivity in the initial hydroboration of the
terminal double bond. As a result, both configurations are formed at C(6). This problem
was overcome using the epimerization process from Scheme 13.34.
The syntheses in Schemes 13.36 to 13.40 are conceptually related. They begin with
symmetric achiral derivatives of meso-2,4-dimethylglutaric acid and utilize various
approaches to the desymmetrization of the meso starting material. In Scheme 13.36
Scheme 13.35. Prelog-Djerassi Lactone Synthesis: W. C. Still and K. R. Shaw a
A
1) O P(OMe) 2
B OH
MeO CHCH , 3 CH 3 B 2 H , 6 TBDMSOCH 2 CH OH
2
–
O CH CH 2 NaH CH 2 H 2 O , OH 2
2
2) LiAlH TBDMSOCH CH 1:1 mixture of CH CH CH 3
CH 4 CH 3 2) LiAlH 3 3
CH 3 3 3) TBDMS Cl 2 3 4 diastereomers
,Celite
C 1) AgCO 3 b
2) epimerization
D
CH CH 3 CH
CH 3 3 3
1) F –
H OTBDMS
CO 2 2) CrO CH 2
O O 3 O O
H H
CH CH
3 3
a. W. C. Still and K. R. Shaw, Tetrahedron Lett., 22, 3725 (1981).
b. Epimerization as in Scheme 13.34.