Page 1227 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1227
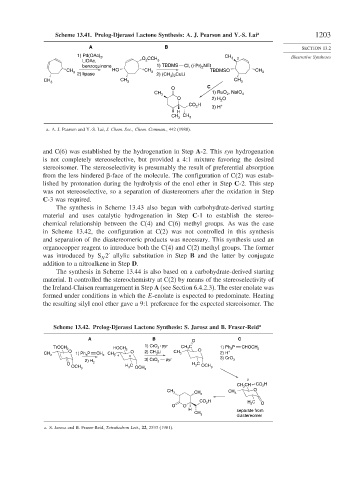
Scheme 13.41. Prelog-Djerassi Lactone Synthesis: A. J. Pearson and Y.-S. Lai a 1203
A B SECTION 13.2
1) Pd(OAc) ,
2 O CCH CH 3 Illustrative Syntheses
LiOAc, 2 3 2
benzoquinone 1) TBDMS Cl, (i-Pr) 2 NEt
CH HO CH TBDMSO CH
3
2) lipase 3 2) (CH 3 2 3
) CuLi
CH 3 CH 3 CH 3
C
O
CH 1) RuO , NaIO
3 2 4
O 2) H O
2
CO H +
2 3) H
H
CH CH 3
3
a. A. J. Pearson and Y.-S. Lai, J. Chem. Soc., Chem. Commun., 442 (1988).
and C(6) was established by the hydrogenation in Step A-2. This syn hydrogenation
is not completely stereoselective, but provided a 4:1 mixture favoring the desired
stereoisomer. The stereoselectivity is presumably the result of preferential absorption
from the less hindered -face of the molecule. The configuration of C(2) was estab-
lished by protonation during the hydrolysis of the enol ether in Step C-2. This step
was not stereoselective, so a separation of diastereomers after the oxidation in Step
C-3 was required.
The synthesis in Scheme 13.43 also began with carbohydrate-derived starting
material and uses catalytic hydrogenation in Step C-1 to establish the stereo-
chemical relationship between the C(4) and C(6) methyl groups. As was the case
in Scheme 13.42, the configuration at C(2) was not controlled in this synthesis
and separation of the diastereomeric products was necessary. This synthesis used an
organocopper reagent to introduce both the C(4) and C(2) methyl groups. The former
was introduced by S 2 allylic substitution in Step B and the latter by conjugate
N
addition to a nitroalkene in Step D.
The synthesis in Scheme 13.44 is also based on a carbohydrate-derived starting
material. It controlled the stereochemistry at C(2) by means of the stereoselectivity of
the Ireland-Claisen rearrangement in Step A (see Section 6.4.2.3). The ester enolate was
formed under conditions in which the E-enolate is expected to predominate. Heating
the resulting silyl enol ether gave a 9:1 preference for the expected stereoisomer. The
Scheme 13.42. Prelog-Djerassi Lactone Synthesis: S. Jarosz and B. Fraser-Reid a
A B O C
1) CrO - pyr CH C
TrOCH 2 3 1) Ph P CHOCH 3
HOCH 2 3 3
O O 2) CH Li CH O +
CH 3 1) Ph P CH CH 3 4 3 3 2) H
3 2 6 3) CrO
2) H 3) CrO 3 pyr 3
O 2 H C
OCH H C OCH 3 OCH 3
3
3 3
2
CH CO H
CH 3 2
CH O
CH 3
CH 3 3
CO 2 H H C O
O O 3
H separate from
CH
3 diastereomer
a. S. Jarosz and B. Fraser-Reid, Tetrahedron Lett., 22, 2533 (1981).