Page 1229 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1229
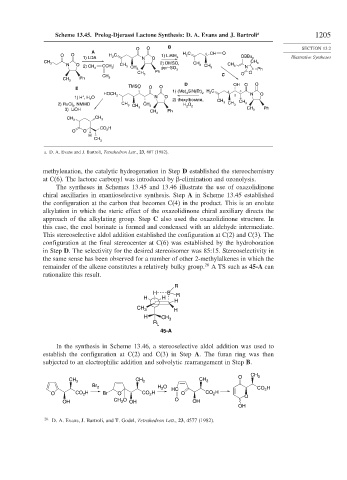
Scheme 13.45. Prelog-Djerassi Lactone Synthesis: D. A. Evans and J. Bartroli a 1205
O O B SECTION 13.2
A O
O O H C 1) LiAlH H C 4 CH OBBu
2
1) LDA 2 N O 4 2 Illustrative Syntheses
CH 3 N O CH 2) DMSO, CH CH CH 3
2) CH
2 CCH I 2 3 CH pyr–SO 3 CH 3 3 N Ph
3 3
CH Ph O O
CH 3 C
CH Ph 3
3
D OH O O
TMSO O O
E H C
HOCH 1) (Me) 3 SiN(Et) 2 2 4 2 N O
+ 2 3
1) H , H O N O
2 2) thexylborane, CH 3 CH
2) RuCl , NMMO CH CH H O CH 3 3
3 3 CH 3 2 2
3) LiOH 3 CH 3 Ph
CH 3 Ph
CH CH
3 3
CO H
O O 2
H
CH 3
a. D. A. Evans and J. Bartroli, Tetrahedron Lett., 23, 807 (1982).
methylenation, the catalytic hydrogenation in Step D established the stereochemistry
at C(6). The lactone carbonyl was introduced by -elimination and ozonolysis.
The syntheses in Schemes 13.45 and 13.46 illustrate the use of oxazolidinone
chiral auxiliaries in enantioselective synthesis. Step A in Scheme 13.45 established
the configuration at the carbon that becomes C(4) in the product. This is an enolate
alkylation in which the steric effect of the oxazolidinone chiral auxiliary directs the
approach of the alkylating group. Step C also used the oxazolidinone structure. In
this case, the enol borinate is formed and condensed with an aldehyde intermediate.
This stereoselective aldol addition established the configuration at C(2) and C(3). The
configuration at the final stereocenter at C(6) was established by the hydroboration
in Step D. The selectivity for the desired stereoisomer was 85:15. Stereoselectivity in
the same sense has been observed for a number of other 2-methylalkenes in which the
28
remainder of the alkene constitutes a relatively bulky group. A TS such as 45-A can
rationalize this result.
R
H B R
H H
H
CH 3 H
H CH 3
R L
45-A
In the synthesis in Scheme 13.46, a stereoselective aldol addition was used to
establish the configuration at C(2) and C(3) in Step A. The furan ring was then
subjected to an electrophilic addition and solvolytic rearrangement in Step B.
CH
O 3
CH
CH 3 CH 3 3
Br 2 H O HC CO 2 H
2
O CO H Br O CO H O CO H O
2
2
2
O O
OH CH 3 OH OH
OH
28
D. A. Evans, J. Bartroli, and T. Godel, Tetrahedron Lett., 23, 4577 (1982).