Page 1228 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1228
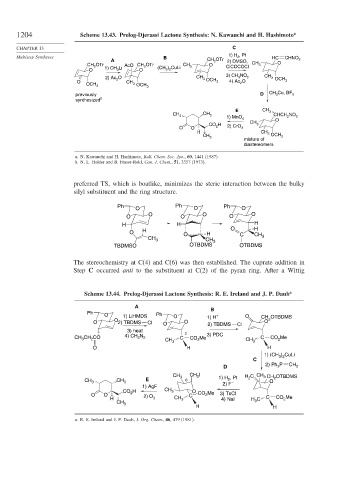
1204 Scheme 13.43. Prelog-Djerassi Lactone Synthesis: N. Kawauchi and H. Hashimoto a
CHAPTER 13 C
, Pt
Multistep Syntheses B 1) H 2 HC CHNO
A CH OTr 2) DMSO, 2
2
CH OTr AcO CH OTr CH O CH 3 O
2 1) CH 3 Li 2 (CH ) CuLi 3 ClCOCOCl
O O 3 2
3) CH NO
2) Ac 2 O CH 3 3 2 CH 3 OCH
O CH OCH 3 4) Ac O 3
OCH 3 2
3 OCH 3
previously D CH 3 Cu, BF 3
synthesized b
E CH 3
CH CH CHCH NO
3 3 2 2
1) MnO 4 O
CH
CO 2 H 2) CrO 3
O O 3
H CH
3
OCH 3
CH 3
mixture of
diastereomers
a. N. Kawauchi and H. Hashimoto, Bull. Chem. Soc. Jpn., 60, 1441 (1987).
b. N. L. Holder and B. Fraser-Reid, Can. J. Chem., 51, 3357 (1973).
preferred TS, which is boatlike, minimizes the steric interaction between the bulky
silyl substituent and the ring structure.
Ph Ph Ph
O O O
O O O
O O O
H H H
O H O H O C H
CH 3
CH 3
CH 3
TBDMSO OTBDMS OTBDMS
The stereochemistry at C(4) and C(6) was then established. The cuprate addition in
Step C occurred anti to the substituent at C(2) of the pyran ring. After a Wittig
Scheme 13.44. Prelog-Djerassi Lactone Synthesis: R. E. Ireland and J. P. Daub a
A
B
Ph O 1) LiHMDS Ph O 1) H + O CH OTBDMS
O O 2) TBDMS Cl O O 2) TBDMS Cl O 2
3) heat 2
N
CH CO 4) CH 2 2 3) PDC
2 2 CH 2
CH 3 C CO Me C CO Me
CH 3 3
O H H
1) (CH ) CuLi
3 2
C
2) Ph P
D 3 CH 2
CH CH I C CH 3 CH OTBDMS
2
2
CH E 3 6 1) H , Pt H 2 2
CH 3 3 2) F – O
1) AgF
CO H CH 3 O
O O 2 2) O C CO Me 3) TsCl
2
H 3 CH 3 4) NaI H 3 C C CO Me
2
CH 3
H H
a. R. E. Ireland and J. P. Daub, J. Org. Chem., 46, 479 (1981).