Page 1231 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1231
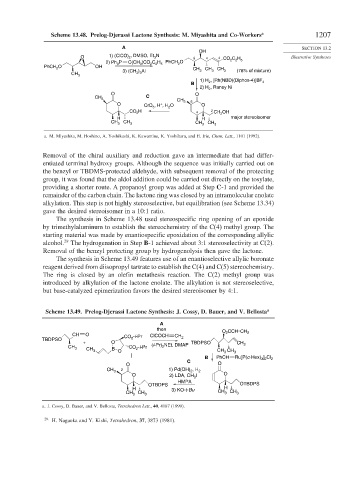
Scheme 13.48. Prelog-Djerassi Lactone Synthesis: M. Miyashita and Co-Workers a 1207
A SECTION 13.2
OH
O 1) (ClCO) , DMSO, Et N 2 4 4 CO C H Illustrative Syntheses
2
3
2) Ph P C(CH 3 )CO C H PhCH 2 O 2 2 5
PhCH O OH 3 2 2 5
2 CH CH
3) (CH ) Al 3 CH 3 3 (78% of mixture)
CH 3 3 3
, [Rh(NBD)(Diphos-4)]BF
1) H 2 4
B
2) H , Raney Ni
2
O O
CH 3 C
CH
O , H , H O 3 6 O
+
CrO 3
2
CO H 4 2 CH OH
2
2
H H major stereoisomer
CH 3 CH 3 CH 3 CH 3
a. M. Miyashita, M. Hoshino, A. Yoshikoshi, K. Kawamine, K. Yoshihara, and H. Irie, Chem. Lett., 1101 (1992).
Removal of the chiral auxiliary and reduction gave an intermediate that had differ-
entiated terminal hydroxy groups. Although the sequence was initially carried out on
the benzyl or TBDMS-protected aldehyde, with subsequent removal of the protecting
group, it was found that the aldol addition could be carried out directly on the tosylate,
providing a shorter route. A propanoyl group was added at Step C-1 and provided the
remainder of the carbon chain. The lactone ring was closed by an intramolecular enolate
alkylation. This step is not highly stereoselective, but equilibration (see Scheme 13.34)
gave the desired stereoisomer in a 10:1 ratio.
The synthesis in Scheme 13.48 used stereospecific ring opening of an epoxide
by trimethylaluminum to establish the stereochemistry of the C(4) methyl group. The
starting material was made by enantiospecific epoxidation of the corresponding allylic
29
alcohol. The hydrogenation in Step B-1 achieved about 3:1 stereoselectivity at C(2).
Removal of the benzyl protecting group by hydrogenolysis then gave the lactone.
The synthesis in Scheme 13.49 features use of an enantioselective allylic boronate
reagent derived from diisopropyl tartrate to establish the C(4) and C(5) stereochemistry.
The ring is closed by an olefin metathesis reaction. The C(2) methyl group was
introduced by alkylation of the lactone enolate. The alkylation is not stereoselective,
but base-catalyzed epimerization favors the desired stereoisomer by 4:1.
Scheme 13.49. Prelog-Djerassi Lactone Synthesis: J. Cossy, D. Bauer, and V. Bellosta a
A
then
O 2 CCH CH 2
CH O CO 2 -i-Pr ClCOCH
TBDPSO CH 2
+ O TBDPSO CH 2
CO 2 -i-Pr (i-Pr) 2 NEt, DMAP
CH 3 B
O CH 3 CH 3
CH 3
B PhCH Ru[P(c-Hex) 3 ] 2 Cl 2
C
O O
2 1) Pd(OH) 2 , H 2
CH 3
O 2) LDA, CH 3 I O
HMPA
OTBDPS OTBDPS
H 3) KO-t-Bu H
CH 3 CH 3
CH 3 CH 3
a. J. Cossy, D. Bauer, and V. Bellosta, Tetrahedron Lett., 40, 4187 (1999).
29
H. Nagaoka and Y. Kishi, Tetrahedron, 37, 3873 (1981).