Page 1234 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1234
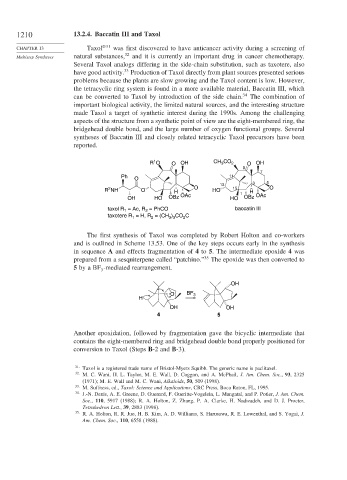
1210 13.2.4. Baccatin III and Taxol
CHAPTER 13 Taxol ®31 was first discovered to have anticancer activity during a screening of
Multistep Syntheses natural substances, 32 and it is currently an important drug in cancer chemotherapy.
Several Taxol analogs differing in the side-chain substitution, such as taxotere, also
33
have good activity. Production of Taxol directly from plant sources presented serious
problems because the plants are slow growing and the Taxol content is low. However,
the tetracyclic ring system is found in a more available material, Baccatin III, which
can be converted to Taxol by introduction of the side chain. 34 The combination of
important biological activity, the limited natural sources, and the interesting structure
made Taxol a target of synthetic interest during the 1990s. Among the challenging
aspects of the structure from a synthetic point of view are the eight-membered ring, the
bridgehead double bond, and the large number of oxygen functional groups. Several
syntheses of Baccatin III and closely related tetracyclic Taxol precursors have been
reported.
1
R O O OH CH 3 CO 2 O OH
9
7
Ph 11
O
3 5
13
2
R NH O H O HO 15 1 H O
OH HO OBz OAc HO OBz OAc
= Ac, R = PhCO baccatin III
taxol R 1 2
taxotere R = H, R = (CH ) CO C
1
2
2
3 3
The first synthesis of Taxol was completed by Robert Holton and co-workers
and is outlined in Scheme 13.53. One of the key steps occurs early in the synthesis
in sequence A and effects fragmentation of 4 to 5. The intermediate epoxide 4 was
35
prepared from a sesquiterpene called “patchino.” The epoxide was then converted to
5 byaBF -mediated rearrangement.
3
OH
O BF 3
H
OH OH
4 5
Another epoxidation, followed by fragmentation gave the bicyclic intermediate that
contains the eight-membered ring and bridgehead double bond properly positioned for
conversion to Taxol (Steps B-2 and B-3).
31 Taxol is a registered trade name of Bristol-Myers Squibb. The generic name is paclitaxel.
32 M. C. Wani, H. L. Taylor, M. E. Wall, D. Coggon, and A. McPhail, J. Am. Chem. Soc., 93, 2325
(1971); M. E. Wall and M. C. Wani, Alkaloids, 50, 509 (1998).
33
M. Suffness, ed., Taxol: Science and Applications, CRC Press, Boca Raton, FL, 1995.
34 J.-N. Denis, A. E. Greene, D. Guenard, F. Gueritte-Vogelein, L. Mangatal, and P. Potier, J. Am. Chem.
Soc., 110, 5917 (1988); R. A. Holton, Z. Zhang, P. A. Clarke, H. Nadizadeh, and D. J. Procter,
Tetrahedron Lett., 39, 2883 (1998).
35
R. A. Holton, R. R. Juo, H. B. Kim, A. D. Williams, S. Harusawa, R. E. Lowenthal, and S. Yogai, J.
Am. Chem. Soc., 110, 6558 (1988).