Page 1238 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1238
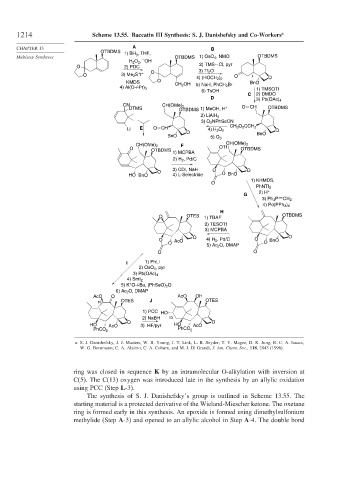
1214 Scheme 13.55. Baccatin III Synthesis: S. J. Danishefsky and Co-Workers a
CHAPTER 13 A B
OTBDMS , THF,
1) BH 3
Multistep Syntheses OTBDMS 1) OsO 4 , NMO OTBDMS
–
H 2 O 2 , OH
O 2) PDC 2) TMS Cl, pyr
+ –
O 3) Me 3 S I O 3) Tf 2 O O 4
4) (HOCH 2 ) 2 O
O
KMDS CH 2 OH 5) NaH, PhCH 2 Br BnO
4) Al(O-i-Pr) 3 1) TMSOTf
6) TsOH
C 2) DMDO
D
3) Pb(OAc) 4
CN CH(OMe) 2
OTMS OTBDMS 1) MeOH, H + O CH OTBDMS
2) LiAlH 4
3) O 2 NPhSeCN
Li E O CH 4) H 2 O 2 CH 3 O 2 CCH 2 O
O BnO
BnO
5) O 3
CH(OMe) 2 F CH(OMe) 2
O OTf OTBDMS
OTBDMS
1) MCPBA
2) H 2 , Pd/C
3) CDI, NaH O O
O
HO BnO 4) L-Selectride O BnO
1) KHMDS,
O
PhNTf 2
2) H +
G
3) Ph 3 P CH 2
4) Pd(PPh 3 ) 4
H
O OTES 1) TBAF OTBDMS
2) TESOTf
3) MCPBA
O O
O AcO 4) H 2 , Pd/C O BnO
O O
5) Ac 2 O, DMAP
O O
I 1) PhLi
2) OsO 4 , pyr
3) Pb(OAc) 4
4) SmI 2
+
5) K O-i-Bu, (PhSeO) 2 O
6) Ac 2 O, DMAP
AcO O AcO OH
OTES J OTES
10
1) PCC HO
2) NaBH 13
O O
HO AcO 3) HF/pyr HO AcO
PhCO 2 PhCO 2
a. S. J. Danishefsky, J. J. Masters, W. B. Young, J. T. Link, L. B. Snyder, T. V. Magee, D. K. Jung, R. C. A. Isaacs,
W. G. Bornmann, C. A. Alaimo, C. A. Coburn, and M. J. Di Grandi, J. Am. Chem. Soc., 118, 2843 (1996).
ring was closed in sequence K by an intramolecular O-alkylation with inversion at
C(5). The C(13) oxygen was introduced late in the synthesis by an allylic oxidation
using PCC (Step L-3).
The synthesis of S. J. Danishefsky’s group is outlined in Scheme 13.55. The
starting material is a protected derivative of the Wieland-Miescher ketone. The oxetane
ring is formed early in this synthesis. An epoxide is formed using dimethylsulfonium
methylide (Step A-3) and opened to an allylic alcohol in Step A-4. The double bond