Page 1243 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1243
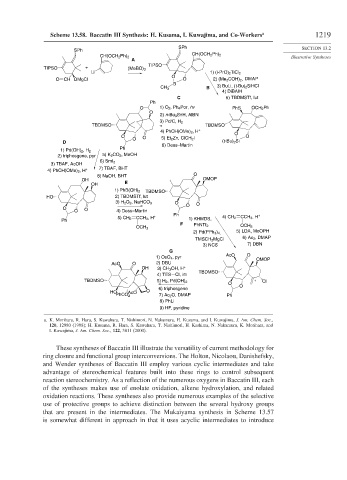
Scheme 13.58. Baccatin III Synthesis: H. Kusama, I. Kuwajima, and Co-Workers a 1219
SPh
SPh SECTION 13.2
CH(OCH 2 Ph) 2
CH(OCH 2 Ph) 2 Illustrative Syntheses
A
TIPSO
TIPSO + (MeBO) 3
Li 1) (i-PrO) 2 TiCl 2
O
O CH OMgCl O 2) (Me 2 COH) 2 , DMAP
B
CH 3 B 3) BuLi, (t-Bu) 2 SiHCl
4) DiBAlH
C 5) TBDMSTf, lut
Ph
O 1) O 2 , Ph 4 Por, hν PhS OCH 2 Ph
O
2) n-Bu 3 SnH, AlBN
3) Pd/C, H 2
TBDMSO TBDMSO
4) PhCH(OMe) 2 , H +
O O 5) Et 2 Zn, ClCH 2 I O O
O
D (t-Bu) 2 Si
6) Dess–Martin
Ph
1) Pd(OH) 2 , H 2
2) triphosgene, pyr 5) K 2 CO 3 , MeOH
6) SmI 2
3) TBAF, AcOH
4) PhCH(OMe) 2 , H + 7) TBAF, BHT
8) NaOH, BHT O
OH OMOP
OH E
1) PhB(OH) 2 TBDMSO
HO 2) TBDMSTf, lut
3) H 2 O 2 , NaHCO 3 O O O
O O
O 4) Dess–Martin
Ph 4) CH 2 CCH 3 , H +
5) CH 2 CCH 3 , H + 1) KHMDS,
Ph
F PhNTf 2
OCH 3 OCH 3
5) LDA, MoOPH
2) Pd(PPh 3 ) 4,
TMSCH 2 MgCl 6) Ac 2 , DMAP
3) NCS 7) DBN
G
AcO O
1) OsO 4 , pyr
OMOP
AcO O 2) DBU
OH 3) CH 3 OH, H +
TBDMSO
4) TES Cl, im
TBDMSO 5) H 2 , Pd(OH) 2 4 Cl
O O
6) triphosgene
HO AcO O
PhCO 2 7) Ac 2 O, DMAP Ph
8) PhLi
9) HF, pyridine
a. K. Morihara, R. Hara, S. Kawahara, T. Nishimori, N. Nakamura, H. Kusama, and I. Kuwajima, J. Am. Chem. Soc.,
120, 12980 (1998); H. Kusama, R. Hara, S. Kawahara, T. Nishimori, H. Kashima, N. Nakamura, K. Morihara, and
I. Kuwajima, J. Am. Chem. Soc., 122, 3811 (2000).
These syntheses of Baccatin III illustrate the versatility of current methodology for
ring closure and functional group interconversions. The Holton, Nicolaou, Danishefsky,
and Wender syntheses of Baccatin III employ various cyclic intermediates and take
advantage of stereochemical features built into these rings to control subsequent
reaction stereochemistry. As a reflection of the numerous oxygens in Baccatin III, each
of the syntheses makes use of enolate oxidation, alkene hydroxylation, and related
oxidation reactions. These syntheses also provide numerous examples of the selective
use of protective groups to achieve distinction between the several hydroxy groups
that are present in the intermediates. The Mukaiyama synthesis in Scheme 13.57
is somewhat different in approach in that it uses acyclic intermediates to introduce