Page 1246 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1246
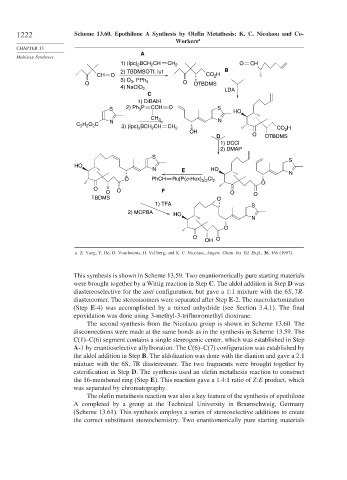
1222 Scheme 13.60. Epothilone A Synthesis by Olefin Metathesis: K. C. Nicolaou and Co-
Workers a
CHAPTER 13
A
Multistep Syntheses
1) (Ipc) 2 BCH CH CH 2 O CH
2
2) TBDMSOTf, lut B
CH O CO H
2
3) O , PPh 3
3
O O OTBDMS
4) NaClO 2 LDA
C
1) DiBAlH
S 2) Ph P CCH O S
3
HO
CH 3 N
N
H O C
C 2 2 2 3) (Ipc) BCH CH CH 2 H
2
2
OH CO 2
D O OTBDMS
1) DCCI
2) DMAP
S
S
HO
N E HO
N
O PhCH Ru[P(c-Hex) ] Cl 2
3 2
O
O
O O F O O
TBDMS O
1) TFA S
2) MCPBA HO
N
O
O O
OH
a. Z. Yang, Y. He, D. Vourloumis, H. Vallberg, and K. C. Nicolaou, Angew. Chem. Int. Ed. Engl., 36, 166 (1997).
This synthesis is shown in Scheme 13.59. Two enantiomerically pure starting materials
were brought together by a Wittig reaction in Step C. The aldol addition in Step D was
diastereoselective for the anti configuration, but gave a 1:1 mixture with the 6S 7R-
diastereomer. The stereoisomers were separated after Step E-2. The macrolactonization
(Step E-4) was accomplished by a mixed anhydride (see Section 3.4.1). The final
epoxidation was done using 3-methyl-3-trifluoromethyl dioxirane.
The second synthesis from the Nicolaou group is shown in Scheme 13.60. The
disconnections were made at the same bonds as in the synthesis in Scheme 13.59. The
C(1)–C(6) segment contains a single stereogenic center, which was established in Step
A-1 by enantioselective allylboration. The C(6)–C(7) configuration was established by
the aldol addition in Step B. The aldolization was done with the dianion and gave a 2:1
mixture with the 6S, 7R diastereomer. The two fragments were brought together by
esterification in Step D. The synthesis used an olefin metathesis reaction to construct
the 16-membered ring (Step E). This reaction gave a 1.4:1 ratio of Z:E product, which
was separated by chromatography.
The olefin metathesis reaction was also a key feature of the synthesis of epothilone
A completed by a group at the Technical University in Braunschweig, Germany
(Scheme 13.61). This synthesis employs a series of stereoselective additions to create
the correct substituent stereochemistry. Two enantiomerically pure starting materials