Page 1251 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1251
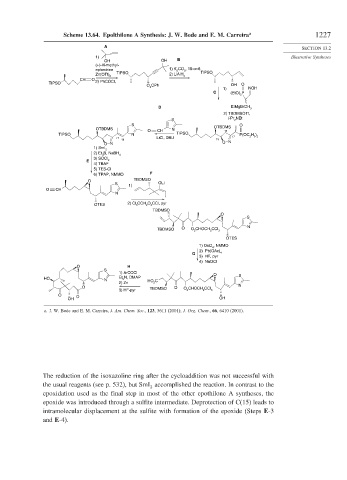
Scheme 13.64. Epolthilone A Synthesis: J. W. Bode and E. M. Carreira a 1227
A
SECTION 13.2
1) Illustrative Syntheses
OH OH B
(+)-N-methyl-
ephedrine 1) K 2 CO , 18-cr-6
3
Zn(OTf) TIPSO 2) LiAlH TIPSO
2 4
CH O
TIPSO 2) PhCOCl, O
O 2 CPh OH
1) NOH
C (EtO) 2 P
D EtMgBrCH 3
2) TBDMSOTf,
S i-Pr NEt
2
S O
OTBDMS N OTBDMS
O CH 16
TIPSO N TIPSO P(OC H )
17 LiCl, DBU 17 2 5 2
18 15 O N
O N
1) SmI 2
2) Et B, NaBH
3 4
3) SOCl
E 2
4) TBAF
5) TES-Cl
6) TPAP, NMMO F
O TBDMSO
S 1) OLi
O CH
N
2) Cl CCH O CCl, pyr
OTES 3 2 2
TBDMSO
O
S
TBDMSO O O CHOCH CCl 3 N
2
2
OTES
1) OsO , NMMO
4
2) Pb(OAc) 4
G
3) HF, pyr
4) NaOCl
O H
S
1) ArCOCl O
HO Et N, DMAP S
N 3 HO C
2) Zn 2
O TBDMSO O O 2 CHOCH 2 CCl 3 N
3) HF-pyr
O O
OH OH
a. J. W. Bode and E. M. Carreira, J. Am. Chem. Soc., 123, 3611 (2001); J. Org. Chem., 66, 6410 (2001).
The reduction of the isoxazoline ring after the cycloaddition was not successful with
the usual reagents (see p. 532), but SmI accomplished the reaction. In contrast to the
2
epoxidation used as the final step in most of the other epothilone A syntheses, the
epoxide was introduced through a sulfite intermediate. Deprotection of C(15) leads to
intramolecular displacement at the sulfite with formation of the epoxide (Steps E-3
and E-4).