Page 1255 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1255
Although each of the epothilone syntheses has its unique features, there are 1231
several recurring themes. Each synthesis uses one or more enantiopure compound as
a starting material. All except the Danishefsky synthesis in Scheme 13.62 utilize the SECTION 13.2
ester bond as a major disconnection. Most also use the C(12)–C(13) double bond Illustrative Syntheses
as a second major disconnection, and several make the synthetic connection by the
alkene (or alkyne) metathesis reaction. Others make the C(11)–C(12) disconnection
and use a Suzuki coupling reaction in the synthetic sense to form the C(10)–C(11)
bond. Wittig reactions figure prominently in the assembly of the thiazole-containing
side chain. The configuration of the isolated stereocenter at C(15) is established by
use of an enantiopure starting material (Schemes 13–59, 13–62, 13–64, and 13–66), an
enantioselective reagent (Schemes 13–60, 13–61, and 13–63), or a kinetic resolution
(Scheme 13–65). The stereochemical issues present are in the C(3)–C(8) segment and
are addressed mainly by aldol reaction stereoselectivity.
13.2.6. Discodermolide
+ -Discodermolide is a natural product isolated from a deep-water sponge found
in the Caribbean Sea. The compound is probably produced by a symbiotic microor-
ganism and isolation is not currently a practical source of the material. Like Taxol and
epothilone A, + -discodermolide is a microtubule stabilizing agent with a promising
profile of antitumor activity. A significant feature of the discodermolide structure is the
three CH -OH-CH triads that establish the configuration of nine stereogenic centers.
3
3
The C(2)–C(4) and C(18)–C(20) triads are syn, anti, whereas the C(10)–C(12) triad
is anti, syn. Seven syntheses are described here. Recently, major elements of two of
these syntheses have been combined to provide sufficient material for Phase I clinical
trials of + -discodermolide.
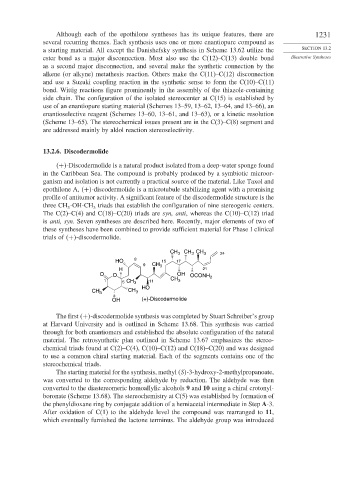
CH 3 CH CH 3 24
3
HO 8 15 17
9 CH 3
H 21
O O OH OCONH 2
1 5 CH 3 11 CH 3
HO
CH 3 CH 3
OH (+)-Discodermolide
The first + -discodermolide synthesis was completed by Stuart Schreiber’s group
at Harvard University and is outlined in Scheme 13.68. This synthesis was carried
through for both enantiomers and established the absolute configuration of the natural
material. The retrosynthetic plan outlined in Scheme 13.67 emphasizes the stereo-
chemical triads found at C(2)–C(4), C(10)–C(12) and C(18)–C(20) and was designed
to use a common chiral starting material. Each of the segments contains one of the
stereochemical triads.
The starting material for the synthesis, methyl S -3-hydroxy-2-methylpropanoate,
was converted to the corresponding aldehyde by reduction. The aldehyde was then
converted to the diastereomeric homoallylic alcohols 9 and 10 using a chiral crotonyl-
boronate (Scheme 13.68). The stereochemistry at C(5) was established by formation of
the phenyldioxane ring by conjugate addition of a hemiacetal intermediate in Step A-3.
After oxidation of C(1) to the aldehyde level the compound was rearranged to 11,
which eventually furnished the lactone terminus. The aldehyde group was introduced