Page 1259 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1259
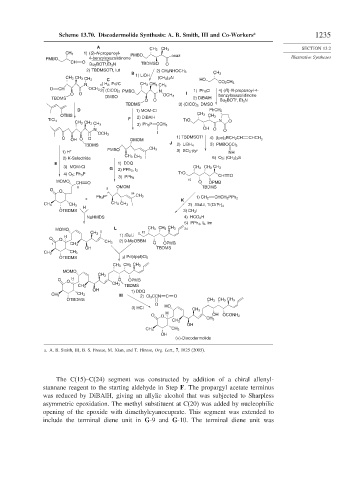
Scheme 13.70. Discodermolide Synthesis: A. B. Smith, III and Co-Workers a 1235
A SECTION 13.2
CH 3 CH 3
1) (S)-N-propanoyl-
CH 3 PMBO oxaz
PMBO 4-benzyloxazolidinone Illustrative Syntheses
CH O
Bu 2 BOTf,Et 3 N TBDMSO O
2) TBDMSOTf, lut 2) CH 3 NHOCH 3
B CH 3
1) LiOH
C HO
CH 3 CH 3 CH 3 (CH 3 ) 3 Al
N 1 ) H 2 , Pd/C CH 3 CH 3 CH 3 CO 2 CH 3
O CH OCH 3 2) (ClCO) 2 PMBO N 1) Ph 3 Cl 4) (R)-N-propanoyl-4-
O O OCH 3 I benzyloxazolidinone
TBDMS DMSO 2) DiBAlH
O O Bu 2 BOTf, Et 3 N
TBDMS 3) (ClCO) 2 , DMSO
D 1) MOM-Cl PhCH 2
OTMS 2) DiBAlH CH 3 CH 3
F
TiCl 4 TrO N O
3) Ph 3 P CCH 3
CH 3 CH 3 CH 3
N OH O O
I
OCH 3
O O O 1) TBDMSOTf 4) (Ipc) 2 BCH 2 CH CHCH
OH OMOM 3
TBDMS J 2) LiBH 4 5) PMBOCCl 3
1) H + PMBO CH 3 3) SO 3 -pyr NH
2) K-Selectride CH 3 CH 3 I 6) O 3 ; (CH 3 ) 2 S
E 1) DDQ
3) MOM-Cl CH 3 CH 3 CH 3
G 2) PPh 3 , I 2
4) O 3 ; Ph 3 P TrO CH O
3) PPh 3
MOMO CH O 15 O OPMB
8 OMOM TBDMS
O O 9 14
+ Ph 3 P + CH 3 K 1) CH 2 CHCH 2 PPh 2
CH 3 CH 3 I
CH 3 CH 3 2) tBuLi, Ti(Oi Pr) 4
H
OTBDMS 3) CH 3 I
NaHMDS 4) HCO 2 H
5) PPh 3 , I 2 , im
MOMO I L CH 3 CH 3 CH 3 24
CH 3 I 15
H 1) tBuLi
O O
CH 3 2) 9-MeOBBN O OPMB
1 CH 3
OH TBDMS
CH 3 CH 3
OTBDMS 3 ) Pd(dppf)Cl 2
CH 3 CH 3 CH 3
MOMO
CH 3
O O H O OPMB
CH 3
CH 3 TBDMS
OH 1) DDQ
CH 3 CH 3 M 2) Cl 3 CCN C O
OTBDMS CH 3 CH 3 CH 3
O
3) HCl HO
CH 3
H
O O OH OCONH 2
CH 3
CH 3
OH
CH 3 CH 3
OH
(+)-Discodermolide
a. A. B. Smith, III, B. S. Freeze, M. Xian, and T. Hirose, Org. Lett., 7, 1825 (2005).
The C(15)–C(24) segment was constructed by addition of a chiral allenyl-
stannane reagent to the starting aldehyde in Step F. The propargyl acetate terminus
was reduced by DiBAlH, giving an allylic alcohol that was subjected to Sharpless
asymmetric epoxidation. The methyl substituent at C(20) was added by nucleophilic
opening of the epoxide with dimethylcyanocuprate. This segment was extended to
include the terminal diene unit in G-9 and G-10. The terminal diene unit was