Page 1264 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1264
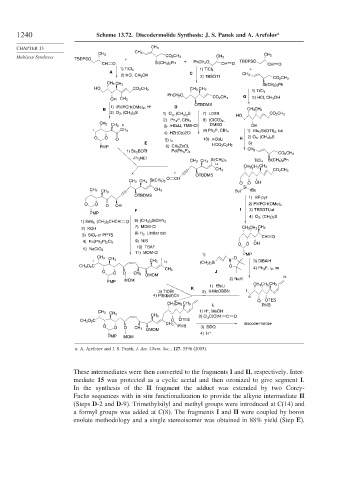
1240 Scheme 13.72. Discodermolide Synthesis: J. S. Panek and A. Arefolov a
CHAPTER 13 CH 3
CH 3
CH 3
Multistep Syntheses TBDPSO + CO 2 CH 3 CH 3 CH 3
CH O Si(CH 3 ) 2 Ph + PhCH 2 O CH O TBDPSO CH O
1) TiCl 4 1) TiCl 4 +
A C
2) HCl, CH 3 OH 2) TBSOTf CH 3
CO 2 CH 3
Si(CH 3 ) 2 Ph
CH 3 CH 3
HO CO 2 CH 3 CH 3 CH 3
1) TiCl 4
PhCH 2 O G
CO 2 CH 3 2) HCl, CH 3 OH
OH CH 3
1) PMPCH(OMe) 2 , H + D OTBDMS
B CH 3 CH 3
2) O 3 , (CH 3 ) 2 S
3 2 HO
1) O 3 , (CH ) S 7) LDBB CO 2 CH 3
2) Ph 3 P, CBr 4 8) (ClCO) 2 ,
6 3) n-BuLi, TMS-Cl OH
CH 3 CH 3 DMSO
1 CH 3 9) Ph 3 P, CBr 4 1) tBu 2 Si(OTf) 2 , lut
4) HZr(Cp)2Cl
O O O 10) n-BuLi H 2) O 3 ; (CH 3 ) 2 S
E 5) I 2 3)
PMP 6) CH 3 ZnCl, HCO 2 C 2 H 5
1) Bu 2 BOTf Pd(Ph 3 P) 4 CH 3
CO 2 CH 3
iPr 2 NEt Si(CH 3 ) 2 Ph
CH 3 CH 3 Si(CH 3 ) 3 TiCl 4
14
CH 3 CH 3 CH 3
CH 3 CO 2 CH 3
7
OTBDMS
O CH
CH 3 CH 3 Si(CH 3 ) 3 OH
O O
Si
CH 3 tBu
CH 3 CH 3 But
OTBDMS
1) HF-pyr
O O 2) PMPCH(OMe) 2
O OH
F I 3) TESOTf,lut
PMP
4) O 3 ; (CH 3 ) 2 S
1) SmI 2 , (CH 3 ) 2 CHCH O 6) (CH 3 ) 3 SiCHN 2
2) KOH 7) MOM-Cl CH 3 CH 3 CH 3
8) H 2 , Lindlar cat
3) SiO 2 or PPTS CH O
9) NIS
4) Ru(Ph 3 P) 2 Cl 2
O O OH
10) TBAF
5) NaClO 2
11) MOM-Cl
1) PMP
CH 3 CH 3 I O
1 CH 3 14 (CH 3 ) 3 Si B 3) DiBAlH
CH 3 O 2 C O
CH 3 4) Ph 3 P, I 2 , im
O O O CH 3 OMOM J
24
2) NaH
PMP MOM
1) tBuLi CH 3 CH 3 CH 3
K
3) TlOEt 2) 9-MeOBBN I
4) Pd(dppf)Cl2
15
O OTES
CH 3 CH 3 CH 3
L PMB
+
1) H , MeOH
CH 3 CH 3
CH 3 2) Cl C(O)N C O
CH 3 O 2 C O OTES 3
CH 3 discodermolide
O O O CH 3 OMOM PMB 3) DDQ
4) H +
PMP MOM
a. A. Arefolov and J. S. Panek, J. Am. Chem. Soc., 127, 5596 (2005).
These intermediates were then converted to the fragments I and II, respectively. Inter-
mediate 15 was protected as a cyclic acetal and then ozonized to give segment I.
In the synthesis of the II fragment the adduct was extended by two Corey-
Fuchs sequences with in situ functionalization to provide the alkyne intermediate II
(Steps D-2 and D-9). Trimethylsilyl and methyl groups were introduced at C(14) and
a formyl groups was added at C(8). The fragments I and II were coupled by boron
enolate methodology and a single stereoisomer was obtained in 88% yield (Step E).