Page 1263 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1263
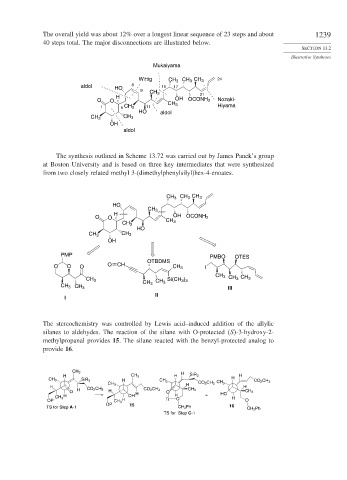
The overall yield was about 12% over a longest linear sequence of 23 steps and about 1239
40 steps total. The major disconnections are illustrated below.
SECTION 13.2
Illustrative Syntheses
Mukaiyama
Wittig CH 3 CH 3 CH 3 24
aldol HO 8 15 17
9
CH 3 21
H
O O OH OCONH 2 Nozaki-
CH 3 Hiyama
1 5 CH 3 11
HO aldol
CH 3 CH 3
OH
aldol
The synthesis outlined in Scheme 13.72 was carried out by James Panek’s group
at Boston University and is based on three key intermediates that were synthesized
from two closely related methyl 3-(dimethylphenylsilyl)hex-4-enoates.
CH 3 CH 3 CH 3
HO
CH 3
H
O O OH OCONH 2
CH 3
CH 3
HO
CH 3 CH 3
OH
PMP PMBO OTES
OTBDMS
O O O O CH CH 3 I
CH 3 CH 3 CH 3
CH 3 Si(CH 3 ) 3
CH 3 CH 3
CH 3 CH 3
III
II
I
The stereochemistry was controlled by Lewis acid–induced addition of the allylic
silanes to aldehydes. The reaction of the silane with O-protected S -3-hydroxy-2-
methylpropanal provides 15. The silane reacted with the benzyl-protected analog to
provide 16.
CH 3 H
H CH 3 H SiR 3 H H
CH 3 SiR 3 H CH 3 CO 2 CH 3
CO 2 CH 3 CH 3
CH 3 H
H H
O H CO 2 CH 3 H CO 2 CH 3 O CH 3 CH 3
H OH H H HO
CH 3 Ti O H
OP CH 3 H O
OP 15
TS for Step A-1 CH 2 Ph 16 CH 2 Ph
TS for Step C-1