Page 1262 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1262
1238 CH 3 CH 3
PMBO CH 3 CH 3 OCH 2 Ph
PMBO PMBO
H C 6 H 11 OCH 2 Ph LiBH 4
CHAPTER 13 CH 3 B
PhCH 2 O O O O OH OH
C 6 H 11 B
Multistep Syntheses CH 3 O C 6 H 11 C 6 H 11 13
TS-1
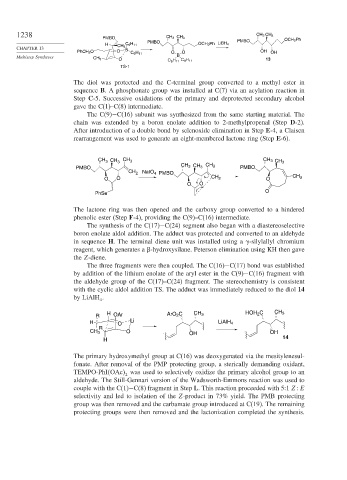
The diol was protected and the C-terminal group converted to a methyl ester in
sequence B. A phosphonate group was installed at C(7) via an acylation reaction in
Step C-5. Successive oxidations of the primary and deprotected secondary alcohol
gave the C(1)–C(8) intermediate.
The C(9)−C(16) subunit was synthesized from the same starting material. The
chain was extended by a boron enolate addition to 2-methylpropenal (Step D-2).
After introduction of a double bond by selenoxide elimination in Step E-4, a Claisen
rearrangement was used to generate an eight-membered lactone ring (Step E-6).
CH 3 CH 3 CH 3 CH 3 CH 3
CH 3 CH 3
PMBO CH 3 PMBO
CH 2 NaIO PMBO
4
O O CH 2 O CH 3
O O
O
PhSe
The lactone ring was then opened and the carboxy group converted to a hindered
phenolic ester (Step F-4), providing the C(9)–C(16) intermediate.
The synthesis of the C(17)−C(24) segment also began with a diastereoselective
boron enolate aldol addition. The adduct was protected and converted to an aldehyde
in sequence H. The terminal diene unit was installed using a -silylallyl chromium
reagent, which generates a -hydroxysilane. Peterson elimination using KH then gave
the Z-diene.
The three fragments were then coupled. The C(16)−C(17) bond was established
by addition of the lithium enolate of the aryl ester in the C(9)−C(16) fragment with
the aldehyde group of the C(17)–C(24) fragment. The stereochemistry is consistent
with the cyclic aldol addition TS. The adduct was immediately reduced to the diol 14
by LiAlH .
4
R H OAr ArO 2 C CH 3 HOH 2 C CH 3
H O Li LiAlH 4
R
CH 3 O OH OH
H 14
The primary hydroxymethyl group at C(16) was deoxygenated via the mesitylenesul-
fonate. After removal of the PMP protecting group, a sterically demanding oxidant,
TEMPO-PhI OAc was used to selectively oxidize the primary alcohol group to an
2
aldehyde. The Still-Gennari version of the Wadsworth-Emmons reaction was used to
couple with the C(1)−C(8) fragment in Step L. This reaction proceeded with 5:1 Z
E
selectivity and led to isolation of the Z-product in 73% yield. The PMB protecting
group was then removed and the carbamate group introduced at C(19). The remaining
protecting groups were then removed and the lactonization completed the synthesis.