Page 1258 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1258
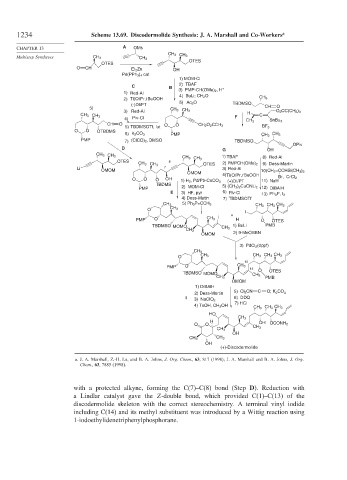
1234 Scheme 13.69. Discodermolide Synthesis: J. A. Marshall and Co-Workers a
CHAPTER 13 A OMs
CH 3 CH 3
Multistep Syntheses CH 3 CH 3
OTES
OTES
O CH Et 2 Zn OH
Pd(PPh 3 ) 4 cat
1) MOM-Cl
2) TBAF
C B
3) PMP-CH(OMe) 2 , H +
1) Red-Al
4) BuLi; CH 2 O
2) Ti(OiPr,tBuOOH CH 3
(-)DIiPT 5) Ac 2 O TBDMSO
5) CH O
3) Red-Al CH 3 CH 3 O 2 CC(CH 3 ) 3
H
CH 3 CH 3 F C
4) Piv-Cl
CH 3 SnBu 3
CH O O O CH 2 O 2 CCH 3
5) TBDMSOTf, lut BF 3
O O OTBDMS
6) K 2 CO 3 PMP CH 3 CH 3
PMP 7) (ClCO) 2 , DMSO TBDMSO
OPiv
D G OH
CH 3 CH 3 1) TBAF 8) Red-Al
CH 3 CH 3
OTES 8
CH 3 CH 3 OTES 2) PMPCH(OMe) 2 9) Dess-Martin
Li 7 3) Red-Al
OMOM 10) CH 2 =CCHSi(CH 3 ) 3
OMOM
4) Ti(OiPr,t BuOOH
O O O OH 1) H 2 , Pd/Pb-CaCO 3 (+)DIiPT 11) NaH Br, CrCl 2
TBDMS
PMP 2) MOM-Cl 5) (CH 3 ) 2 CuCNLi 2 12) DiBAlH
E 3) HF, pyr 6) Piv-Cl
13) Ph 3 P, I 2
4) Dess-Matin 7) TBDMSOTf
CH 3 5) Ph 3 P=CCH 3 CH 3 CH 3 CH 3
O CH 3
I I
I +
PMP O CH 3 H O OTES
TBDMSO MOMO 1) BuLi PMB
CH 3 CH 3 2) 9-MeOBBN
OMOM
3) PdCl 2 (dppf)
CH 3
O CH 3 CH 3 CH 3 CH 3
15
PMP O CH 3 14
TBDMSO MOMO O OTES
CH 3
CH 3 PMB
OMOM
1) DiBAlH
5) Cl 3 CN C
2) Dess-Martin O; K 2 CO 3
I 6) DDQ
3) NaClO 2
7) HCl
4) TsOH, CH 3 OH
CH 3 CH 3 CH 3
HO
CH 3
H OH
O O OCONH 2
CH 3
CH 3
OH
CH 3 CH 3
OH
(+)-Discodermolide
a. J. A. Marshall, Z.-H. Lu, and B. A. Johns, J. Org. Chem., 63, 817 (1998); J. A. Marshall and B. A. Johns, J. Org.
Chem., 63, 7885 (1998).
with a protected alkyne, forming the C(7)–C(8) bond (Step D). Reduction with
a Lindlar catalyst gave the Z-double bond, which provided C(1)–C(13) of the
discodermolide skeleton with the correct stereochemistry. A terminal vinyl iodide
including C(14) and its methyl substituent was introduced by a Wittig reaction using
1-iodoethylidenetriphenylphosphorane.