Page 1253 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1253
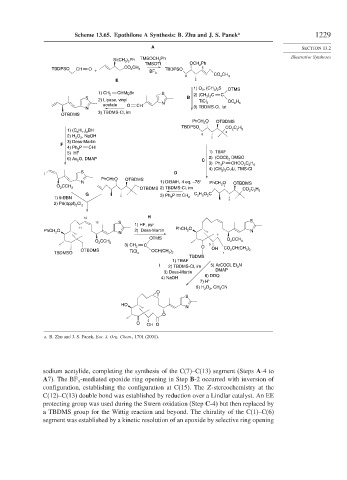
Scheme 13.65. Epothilone A Synthesis: B. Zhu and J. S. Panek a 1229
A SECTION 13.2
Si(CH ) Ph TMSOCH Ph Illustrative Syntheses
2
3 2
TMSOTf OCH Ph
CH 2
TBDPSO CH O + CO 2 3 BF TBDPSO 5
3 CH
8 CO 2 3
E
1) O , (CH ) S OTMS
3 3 2
CHMgBr
1) CH 2 S 2) (CH ) C C
S B 3 2
2) Lipase, vinyl TiCl 4 OC H
acetate O CH N 2 5
N 3) TBDMS-Cl, lut
3) TBDMS-Cl, im
OTBDMS
PhCH O OTBDMS
2
TBDPSO CO C H
1) (C H ) BH 3 2 2 5
6
11 2
O , NaOH 8
2
2) H 2
3) Dess-Martin
F
4) Ph 3 P CHI
5) HF 1) TBAF
O, DMAP 2) (COCl) DMSO
C
6) Ac 2 2
3) Ph P CHCO C H
3 2 2 5
4) (CH ) CuLi, TMS-Cl
S 3 2
I D
PhCH2O OTBDMS
N 1) DiBAlH, 4 eq, –78° O OTBDMS
PhCH 2
O CCH
2 3 OTBDMS 2) TBDMS-Cl, im CO 2 C H 5
2
G 3) Ph P CH C H O C
5
2
2
1) 9-BBN 3 2
2) Pd(dppf) Cl
2 2
12 H
13 S S
1) HF, pyr
11 O
O 2) Dess-Martin PhCH 2
N
PhCH 2 N
2
OTMS CCH
CCH O 2
O 2 3
3
3) CH C 3
2 O OH CO CH(CH )
OTBDMS TiCl ) 2 3 2
TBDMSO 4 OCH(CH 3 2 1
TBDMS
1) TBAF
I 2) TBDMS-Cl, im 5) ArCOCl, Et 3 N
DMAP
3) Dess-Martin
6) DDQ
4) NaOH
7) H +
8) H O , CH CN
O 2 2 3
S
HO
N
O
O OH O
a. B. Zhu and J. S. Panek, Eur. J. Org. Chem., 1701 (2001).
sodium acetylide, completing the synthesis of the C(7)–C(13) segment (Steps A-4 to
A7). The BF -mediated epoxide ring opening in Step B-2 occurred with inversion of
3
configuration, establishing the configuration at C(15). The Z-stereochemistry at the
C(12)–C(13) double bond was established by reduction over a Lindlar catalyst. An EE
protecting group was used during the Swern oxidation (Step C-4) but then replaced by
a TBDMS group for the Wittig reaction and beyond. The chirality of the C(1)–C(6)
segment was established by a kinetic resolution of an epoxide by selective ring opening