Page 1250 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1250
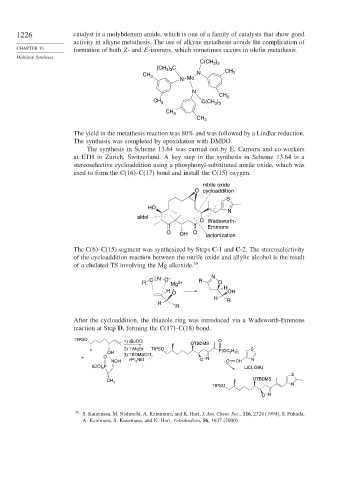
1226 catalyst is a molybdenum amide, which is one of a family of catalysts that show good
activity in alkyne metathesis. The use of alkyne metathesis avoids the complication of
CHAPTER 13 formation of both Z- and E-isomers, which sometimes occurs in olefin metathesis.
Multistep Syntheses
C(CH )
3 3
(CH ) C CH
3 3
CH 3 N 3
N Mo
N
CH 3
CH 3 C(CH )
3 3
CH 3
CH 3
The yield in the metathesis reaction was 80% and was followed by a Lindlar reduction.
The synthesis was completed by epoxidation with DMDO.
The synthesis in Scheme 13.64 was carried out by E. Carreira and co-workers
at ETH in Zurich, Switzerland. A key step in the synthesis in Scheme 13.64 is a
stereoselective cycloaddition using a phosphonyl-substituted nitrile oxide, which was
used to form the C(16)–C(17) bond and install the C(15) oxygen.
nitrile oxide
O cycloaddition
S
HO
N
aldol
O Wadsworth-
Emmons
O OH O lactonization
The C(6)–C(15) segment was synthesized by Steps C-1 and C-2. The stereoselectivity
of the cycloaddition reaction between the nitrile oxide and allylic alcohol is the result
of a chelated TS involving the Mg alkoxide. 39
C N O – R N
R Mg 2+ O
H O H OH
H
H R
R
After the cycloaddition, the thiazole ring was introduced via a Wadsworth-Emmons
reaction at Step D, forming the C(17)–C(18) bond.
TIPSO
1) t BuOCl O
OTBDMS
2) TtMgBr TIPSO S
OH 3) TBDMSOTf, P(OC H )
2
5 2
+ O
NOH i-Pr NEt O N O CH N
2
P
(EtO) 2 LiCl, DBU
S
OTBDMS
CH 3
TIPSO N
O N
39
S. Kanemasa, M. Nishiuchi, A. Kamimura, and K. Hori, J. Am. Chem. Soc., 116, 2324 (1994); S. Fukuda,
A. Kanimura, S. Kanemasa, and K. Hori, Tetrahedron, 56, 1637 (2000).