Page 1254 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1254
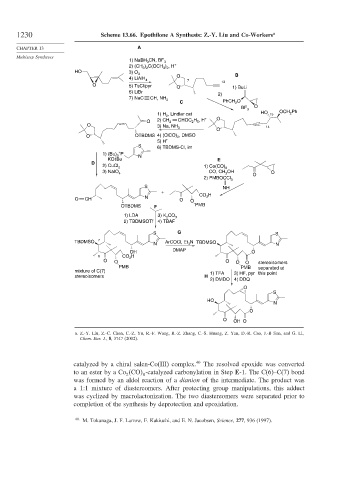
1230 Scheme 13.66. Epothilone A Synthesis: Z.-Y. Liu and Co-Workers a
CHAPTER 13 A
Multistep Syntheses
1) NaBH CN, BF
3 3
2) (CH 3 ) 2 C(OCH 3 ) 2 , H +
HO 3) O 3 B
4) LiAlH 4 O 7
O 5) TsCl/pyr O 13 1) BuLi
6) LiBr
2)
7) NaC CH, NH 3
C PhCH O
2
BF 3 O
1) H , Lindlar cat HO 15 OCH 2 Ph
2 O
O 2) CH 2 CHOC H , H +
2
5
O 3) Na, NH
3 14
O
O OTBDMS 4) (ClCO) 2 , DMSO
5) H +
S 6) TBDMS-Cl, im
+
1) (Bu) P N
3
KOt Bu E
D
2) CuCl 2 1) Co(CO) 8
3) NaIO 4 CO, CH OH O O
3
2) PMBOCCl 3
S NH
+
2
N CO H
O CH O O
OTBDMS F PMB
1) LDA 3) K 2 CO 3
2) TBDMSOTf 4) TBAF
S G S
TBDMSO 7 N TBDMSO
N ArCOCl, Et 3 N
DMAP
OH O
6 CO 2 H
O O O O O stereoisomers
PMB PMB separated at
mixture of C(7) 1) TFA 3) HF, pyr this point
stereoisomers H
2) DMDO 4) DDQ
O
S
HO
N
O
O OH O
a. Z.-Y. Liu, Z.-C. Chen, C.-Z. Yu, R.-F. Wang, R.-Z. Zhang, C.-S. Huang, Z. Yan, D.-R. Cao, J.-B Sun, and G. Li,
Chem. Eur. J., 8, 3747 (2002).
catalyzed by a chiral salen-Co(III) complex. 40 The resolved epoxide was converted
to an ester by a Co CO -catalyzed carbonylation in Step E-1. The C(6)–C(7) bond
2 8
was formed by an aldol reaction of a dianion of the intermediate. The product was
a 1:1 mixture of diastereomers. After protecting group manipulations, this adduct
was cyclized by macrolactonization. The two diastereomers were separated prior to
completion of the synthesis by deprotection and epoxidation.
40
M. Tokunaga, J. F. Larrow, F. Kakiuchi, and E. N. Jacobsen, Science, 277, 936 (1997).