Page 1249 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1249
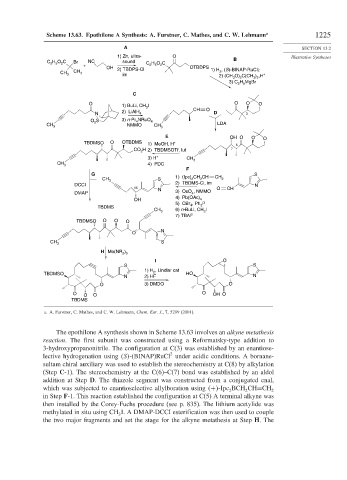
Scheme 13.63. Epothilone A Synthesis: A. Furstner, C. Mathes, and C. W. Lehmann a 1225
A SECTION 13.2
1) Zn, ultra- O Illustrative Syntheses
C H O C Br NC sound H O C B
2
5
2
+ OH C 2 5 2 OTBDPS
CH 2) TBDPS-Cl 1) H 2 , (S)-BINAP-RuCl2
im 2) (CH O) C(CH ) ,H +
CH 3 3
3
2
3 2
3) C H MgBr
5
2
C
O O O O
1) BuLi, CH I
3
CH O
N 2) LiAlH 4 D 3
O S 3) n-Pr NRuO 4
4
CH 2 NMMO CH LDA
3 3
E OH O O O
TBDMSO O OTBDMS 1) MeOH, H + 6
H 2) TBDMSOTf, lut 7
CO 2
3) H + CH
CH 4) PDC 3
3
F
G S
CH 3 S 1) (Ipc) 2 CH CH CH 2
2
DCCI 2) TBDMS-Cl, im N
15 O CH
N 3) OsO , NMMO
DMAP 4
4) Pb(OAc)
OH 4
5) CBr , Ph P
TBDMS 4 3
CH 3 6) n-BuLi, CH 3 I
7) TBAF
TBDMSO O O O
O N
CH 3 S
)
H Mo(NR 2 3
I O
S S
1) H , Lindlar cat
TBDMSO 2 HO N
N 2) HF
O 3) DMDO O
O O O O OH O
TBDMS
a. A. Furstner, C. Mathes, and C. W. Lehmann, Chem. Eur. J., 7, 5299 (2001).
The epothilone A synthesis shown in Scheme 13.63 involves an alkyne metathesis
reaction. The first subunit was constructed using a Reformatsky-type addition to
3-hydroxypropanonitrile. The configuration at C(3) was established by an enantiose-
2
lective hydrogenation using S -(BINAP)RuCl under acidic conditions. A bornane-
sultam chiral auxiliary was used to establish the stereochemistry at C(8) by alkylation
(Step C-1). The stereochemistry at the C(6)–C(7) bond was established by an aldol
addition at Step D. The thiazole segment was constructed from a conjugated enal,
which was subjected to enantioselective allylboration using + -Ipc BCH CH=CH
2 2 2
in Step F-1. This reaction established the configuration at C(5) A terminal alkyne was
then installed by the Corey-Fuchs procedure (see p. 835). The lithium acetylide was
methylated in situ using CH I. A DMAP-DCCI esterification was then used to couple
3
the two major fragments and set the stage for the alkyne metathesis at Step H. The