Page 1248 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1248
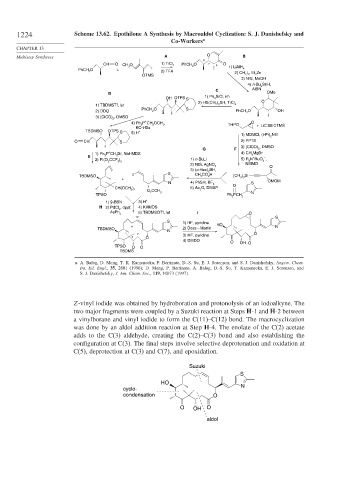
1224 Scheme 13.62. Epothilone A Synthesis by Macroaldol Cyclization: S. J. Danishefsky and
Co-Workers a
CHAPTER 13
Multistep Syntheses A O B
8
CH O O 1) TiCl 4 PhCH O 7 O
CH 3 2 6 1) LiAlH
PhCH O + 4
2 2) TFA 2) CH I , Et Zn
OTMS 2 2 2
3) NIS, MeOH
SnH,
4) n-Bu 3
C AlBN
D OMe
OH OTPS S 1) Ph SiCl, im
3
) SH, TiCl O
1) TBDMSTf, lut 2) HS(CH 2 3 4
O S
2) DDQ PhCH 2 PhCH 2 O OH
3) (ClCO) , DMSO
2
+
P CH OCH , O
4) Ph 3
2 3 THPO + LiC CTMS
KO-t-Bu
TBDMSO OTPS S +
5) H
1) MOMCl, (i-Pr) NEt
2
O CH S 2) PPTS
3) (ClCO) , DMSO
G F 2
+
P CH Br, NaHMDS 4) CH MgBr
1) Ph 3 3 3
E + –
CCF ) 1) n-BuLi 5) R N RuO ,
2) PI(O 2 4 4
3 2
NMMO
2) NIS, AgNO 3 O
3) (c-Hex) BH,
2
TBDMSO I S CH 3 CO H (CH 3 3 ) Si
2
+ OMOM
N 4) PhSH, BF 3 S
CH(OCH ) 5) Ac O, DMAP O
3 2 O CCH 2
TPSO 2 3 Ph 2 PCH N
2
1) 9-BBN 3) H +
, dppf, 4) KHMDS
H 2) PdCl 2
5) TBDMSOTf, lut
AsPh 3 I O
12 S
S 1) HF, pyridine
11 HO N
TBDMSO 2) Dess – Martin
N
2 3) HF, pyridine O
3 O
4) DMDO O OH O
TPSO O O
TBDMS
a. A. Balog, D. Meng, T. K. Kamenecka, P. Bertinato, D.-S. Su, E. J. Sorensen, and S. J. Danishefsky, Angew. Chem.
Int. Ed. Engl., 35, 2801 (1996); D. Meng, P. Bertinato, A. Balog, D.-S. Su, T. Kamenecka, E. J. Sorensen, and
S. J. Danishefsky, J. Am. Chem. Soc., 119, 10073 (1997).
Z-vinyl iodide was obtained by hydroboration and protonolysis of an iodoalkyne. The
two major fragments were coupled by a Suzuki reaction at Steps H-1 and H-2 between
a vinylborane and vinyl iodide to form the C(11)–C(12) bond. The macrocyclization
was done by an aldol addition reaction at Step H-4. The enolate of the C(2) acetate
adds to the C(3) aldehyde, creating the C(2)–C(3) bond and also establishing the
configuration at C(3). The final steps involve selective deprotonation and oxidation at
C(5), deprotection at C(3) and C(7), and epoxidation.
Suzuki
S
HO
cyclo- N
condensation O
O OH O
aldol