Page 1247 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1247
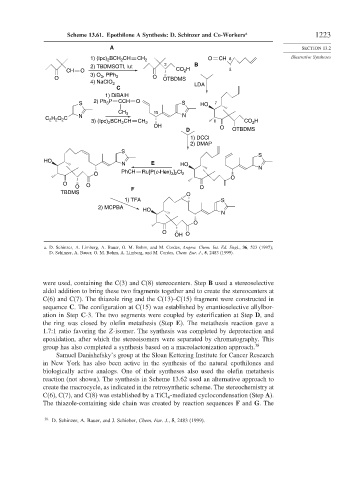
Scheme 13.61. Epothilone A Synthesis: D. Schinzer and Co-Workers a 1223
A SECTION 13.2
BCH CH CH O CH Illustrative Syntheses
1) (Ipc) 2 2 2 8
3 B
2) TBDMSOTf, lut
CH O CO 2 H
3) O , PPh
O 3 3 O OTBDMS
4) NaClO 2
LDA
C
1) DiBAlH
S 2) Ph P CCH O S HO 7
3
CH 3 15
C H O C N 3) (Ipc) BCH CH CH N 6 CO 2 H
2 2
2
2
2
2
OH O
D OTBDMS
1) DCCI
2) DMAP
S
S
HO
N E HO
N
PhCH ] Cl
O Ru[P(c-Hex) 3 2 2
O
O
O O F O
TBDMS O
1) TFA S
2) MCPBA
HO
N
O
O O
OH
a. D. Schinzer, A. Limberg, A. Bauer, O. M. Bohm, and M. Cordes, Angew. Chem. Int. Ed. Engl., 36, 523 (1997);
D. Schinzer, A. Bauer, O. M. Bohm, A. Limberg, and M. Cordes, Chem. Eur. J., 5, 2483 (1999).
were used, containing the C(3) and C(8) stereocenters. Step B used a stereoselective
aldol addition to bring these two fragments together and to create the stereocenters at
C(6) and C(7). The thiazole ring and the C(13)–C(15) fragment were constructed in
sequence C. The configuration at C(15) was established by enantioselective allylbor-
ation in Step C-3. The two segments were coupled by esterification at Step D, and
the ring was closed by olefin metathesis (Step E). The metathesis reaction gave a
1.7:1 ratio favoring the Z-isomer. The synthesis was completed by deprotection and
epoxidation, after which the stereoisomers were separated by chromatography. This
group has also completed a synthesis based on a macrolactonization approach. 38
Samuel Danishefsky’s group at the Sloan Kettering Institute for Cancer Research
in New York has also been active in the synthesis of the natural epothilones and
biologically active analogs. One of their syntheses also used the olefin metathesis
reaction (not shown). The synthesis in Scheme 13.62 used an alternative approach to
create the macrocycle, as indicated in the retrosynthetic scheme. The stereochemistry at
C(6), C(7), and C(8) was established by a TiCl -mediated cyclocondensation (Step A).
4
The thiazole-containing side chain was created by reaction sequences F and G. The
38
D. Schinzer, A. Bauer, and J. Schieber, Chem. Eur. J., 5, 2483 (1999).