Page 1252 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1252
1228 –
F
CHAPTER 13 TBDMSO O
OTBDMS
Multistep Syntheses
SOCl 2 TBAF
O O
OH OH S
OH
O
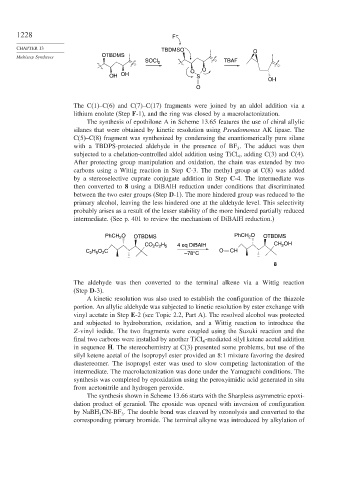
The C(1)–C(6) and C(7)–C(17) fragments were joined by an aldol addition via a
lithium enolate (Step F-1), and the ring was closed by a macrolactonization.
The synthesis of epothilone A in Scheme 13.65 features the use of chiral allylic
silanes that were obtained by kinetic resolution using Pseudomonas AK lipase. The
C(5)–C(8) fragment was synthesized by condensing the enantiomerically pure silane
with a TBDPS-protected aldehyde in the presence of BF . The adduct was then
3
subjected to a chelation-controlled aldol addition using TiCl , adding C(3) and C(4).
4
After protecting group manipulation and oxidation, the chain was extended by two
carbons using a Wittig reaction in Step C-3. The methyl group at C(8) was added
by a stereoselective cuprate conjugate addition in Step C-4. The intermediate was
then converted to 8 using a DiBAlH reduction under conditions that discriminated
between the two ester groups (Step D-1). The more hindered group was reduced to the
primary alcohol, leaving the less hindered one at the aldehyde level. This selectivity
probably arises as a result of the lesser stability of the more hindered partially reduced
intermediate. (See p. 401 to review the mechanism of DiBAlH reduction.)
PhCH O OTBDMS PhCH O OTBDMS
2
2
CO C H 4 eq DiBAlH CH 2 OH
2 2 5
C H O C –78°C O CH
2 5
2
8
The aldehyde was then converted to the terminal alkene via a Wittig reaction
(Step D-3).
A kinetic resolution was also used to establish the configuration of the thiazole
portion. An allylic aldehyde was subjected to kinetic resolution by ester exchange with
vinyl acetate in Step E-2 (see Topic 2.2, Part A). The resolved alcohol was protected
and subjected to hydroboration, oxidation, and a Wittig reaction to introduce the
Z-vinyl iodide. The two fragments were coupled using the Suzuki reaction and the
final two carbons were installed by another TiCl -mediated silyl ketene acetal addition
4
in sequence H. The stereochemistry at C(3) presented some problems, but use of the
silyl ketene acetal of the isopropyl ester provided an 8:1 mixture favoring the desired
diastereomer. The isopropyl ester was used to slow competing lactonization of the
intermediate. The macrolactonization was done under the Yamaguchi conditions. The
synthesis was completed by epoxidation using the peroxyimidic acid generated in situ
from acetonitrile and hydrogen peroxide.
The synthesis shown in Scheme 13.66 starts with the Sharpless asymmetric epoxi-
dation product of geraniol. The epoxide was opened with inversion of configuration
by NaBH CN-BF . The double bond was cleaved by ozonolysis and converted to the
3
3
corresponding primary bromide. The terminal alkyne was introduced by alkylation of