Page 1256 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1256
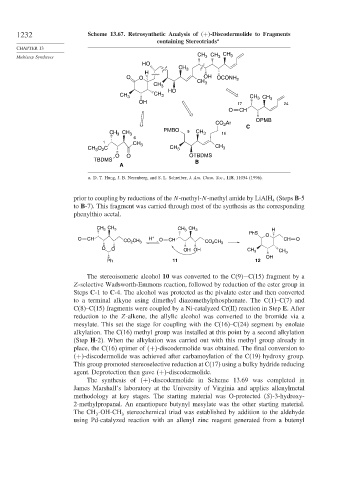
1232 Scheme 13.67. Retrosynthetic Analysis of + -Discodermolide to Fragments
containing Stereotriads a
CHAPTER 13
CH CH CH 3
Multistep Syntheses 3 3
HO
CH 3
H
O O OH OCONH
CH 2
CH 3 3
HO
CH 3 CH 3 CH 3 CH
OH 17 3 24
O CH
OPMB
CO Ar C
2
CH CH 3 PMBO 9 CH 3 16
3
6
1 CH 3
CH 3 O C CH 3 CH 3
2
O O OTBDMS
TBDMS B
A
a. D. T. Hung, J. B. Nerenberg, and S. L. Schreiber, J. Am. Chem. Soc., 118, 11054 (1996).
prior to coupling by reductions of the N-methyl-N-methyl amide by LiAlH (Steps B-5
4
to B-7). This fragment was carried through most of the synthesis as the corresponding
phenylthio acetal.
CH 3 CH 3 CH 3 CH 3 H
PhS O
O CH H + O CH CH O
CO 2 CH 3 CO 2 CH 3
O O OH OH CH 3 CH 3
OH
Ph 11 12
The stereoisomeric alcohol 10 was converted to the C(9)−C(15) fragment by a
Z-selective Wadsworth-Emmons reaction, followed by reduction of the ester group in
Steps C-1 to C-4. The alcohol was protected as the pivalate ester and then converted
to a terminal alkyne using dimethyl diazomethylphosphonate. The C(1)–C(7) and
C(8)–C(15) fragments were coupled by a Ni-catalyzed Cr(II) reaction in Step E. After
reduction to the Z-alkene, the allylic alcohol was converted to the bromide via a
mesylate. This set the stage for coupling with the C(16)–C(24) segment by enolate
alkylation. The C(16) methyl group was installed at this point by a second alkylation
(Step H-2). When the alkylation was carried out with this methyl group already in
place, the C(16) epimer of + -discodermolide was obtained. The final conversion to
+ -discodermolide was achieved after carbamoylation of the C(19) hydroxy group.
This group promoted stereoselective reduction at C(17) using a bulky hydride reducing
agent. Deprotection then gave + -discodermolide.
The synthesis of + -discodermolide in Scheme 13.69 was completed in
James Marshall’s laboratory at the University of Virginia and applies allenylmetal
methodology at key stages. The starting material was O-protected S -3-hydroxy-
2-methylpropanal. An enantiopure butynyl mesylate was the other starting material.
The CH -OH-CH stereochemical triad was established by addition to the aldehyde
3 3
using Pd-catalyzed reaction with an allenyl zinc reagent generated from a butenyl