Page 1260 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1260
1236 introduced by CrCl -mediated addition in Step G-10, followed by base-induced
2
elimination from the -hydroxysilane.
CHAPTER 13 The two major subunits were coupled by a Suzuki reaction in Step H-3. The
Multistep Syntheses synthesis was then completed by reductive opening of the 1,3-dioxane ring, oxidation
of the terminal alcohol to the carboxylic acid, carbamoylation, deprotection, and
lactonization.
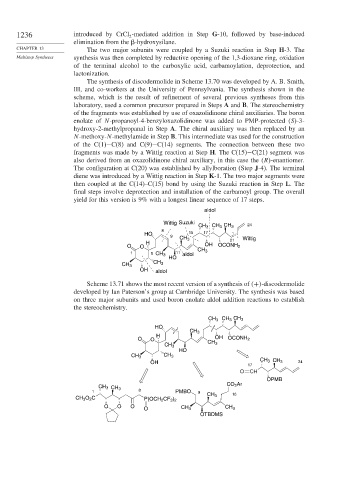
The synthesis of discodermolide in Scheme 13.70 was developed by A. B. Smith,
III, and co-workers at the University of Pennsylvania. The synthesis shown in the
scheme, which is the result of refinement of several previous syntheses from this
laboratory, used a common precursor prepared in Steps A and B. The stereochemistry
of the fragments was established by use of oxazolidinone chiral auxiliaries. The boron
enolate of N-propanoyl-4-benzyloxazolidinone was added to PMP-protected S -3-
hydroxy-2-methylpropanal in Step A. The chiral auxiliary was then replaced by an
N-methoxy-N-methylamide in Step B. This intermediate was used for the construction
of the C(1)−C(8) and C(9)−C(14) segments. The connection between these two
fragments was made by a Wittig reaction at Step H. The C(15)−C(21) segment was
also derived from an oxazolidinone chiral auxiliary, in this case the R -enantiomer.
The configuration at C(20) was established by allylboration (Step J-4). The terminal
diene was introduced by a Wittig reaction in Step K-1. The two major segments were
then coupled at the C(14)–C(15) bond by using the Suzuki reaction in Step L. The
final steps involve deprotection and installation of the carbamoyl group. The overall
yield for this version is 9% with a longest linear sequence of 17 steps.
aldol
Wittig Suzuki 24
CH 3 CH 3 CH 3
8
HO 9 15 17
CH 3 21 Wittig
H OH
O O OCONH 2
CH 3
1 5 CH 3 11 aldol
HO
CH 3
CH 3
OH aldol
Scheme 13.71 shows the most recent version of a synthesis of + -discodermolide
developed by Ian Paterson’s group at Cambridge University. The synthesis was based
on three major subunits and used boron enolate aldol addition reactions to establish
the stereochemistry.
CH 3 CH 3 CH 3
HO
CH 3
H
O O OH OCONH 2
CH 3
CH 3
HO
CH 3 CH 3
OH CH 3 CH 3 24
17
O CH
OPMB
CO 2 Ar
CH 3 CH 3
1 8 PMBO 9
CH 3 16
CH 3 O 2 C
P(OCH 2 CF 3 ) 2
O O O
O CH 3 CH 3
OTBDMS