Page 1257 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1257
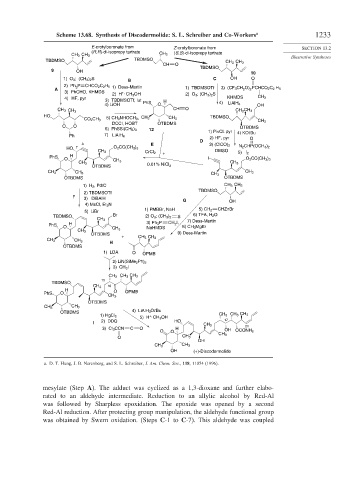
Scheme 13.68. Synthesis of Discodermolide: S. L. Schreiber and Co-Workers a 1233
E-crotylboronate from Z-crotylboronate from SECTION 13.2
(R,R)-di-isopropy tartrate (S,S)-di-isopropy tartrate
CH 3 CH 3 CH 3 Illustrative Syntheses
TBDMSO TBDMSO
CH O CH 3 CH 3
TBDMSO
9 OH
10
1) O 3 ; (CH 3 ) 2 S C OH O
B
P
2) Ph 3 CHCO 2 C 2 H 5 1) Dess-Martin 1) TBDMSOTf
A 3) (CF 3 CH O) PCHCO 2 C 2 H 5
2
2
3) PhCHO, KHMDS
+,
2) H CH 3 OH 2) O 3 ; (CH 3 ) 2 S
4) HF, pyr KHMDS CH 3
3) TBDMSOTf, lut H
4) LiOH PhS O 4) LiAlH 4 OH
CH O
CH 3 CH 3 CH 3
CH 3
HO 5) CH 3 NHOCH 3 , TBDMSO
CO 2 CH 3 CH 3 CH 3
CH 3
DCCI, HOBT OTBDMS
O O OTBDMS
6) PhSSi(CH 3 ) 3 12
1) PivCl. pyr 4) KOt Bu
Ph 7) LiAlH 4 2) HF, pyr O
D
8 E 3) (ClCO) 2
HO 7 O 2 CC(CH 3 ) 3 N 2 CHP(OCH 3 ) 2
CH 3 DMSO 5)
CrCl 2 + I 2
PhS O H I
CH 3 O 2 CC(CH 3 ) 3
CH 3 0.01% NiCl CH 3
OTBDMS 2
CH 3 CH 3 CH 3
CH 3
OTBDMS OTBDMS
1) H 2 , Pd/C CH 3 CH 3
TBDMSO
2) TBDMSOTf
F 3) DiBAlH
G OH
4) MsCl, Et 3 N
1) PMBBr, NaH CHZnBr
5) LiBr 5) CH 2
TBDMSO Br 2) O 3 ; (CH 3 ) 2 S 6) TFA, H 2 O
CH 3 7) Dess-Martin
2
PhS O H 3) Ph 3 P CH I, 8) CH 3 MgBr
CH 3 NaHMDS
CH 3 9) Dess-Martin
OTBDMS
+ CH 3 CH 3
CH 3 CH 3
H
OTBDMS
1) LDA O OPMB
2) LiN(SiMe 2 Ph) 2
3) CH 3 I
CH 3 CH 3 CH 3
15
TBDMSO
CH 3 16
H O
PhS O OPMB
CH 3
OTBDMS
CH 3 CH 3
4) LiAlH 3 Ot Bu
OTBDMS
1) HgCl 2 +, CH 3 CH 3 CH 3
5) H CH 3 OH
2) DDQ HO 17
I
CH 3 21
3) Cl 3 CCN C O H OH
O O OCONH 2
CH 3
O CH 3
OH
CH 3 CH 3
OH (+)-Discodermolide
a. D. T. Hung, J. B. Nerenberg, and S. L. Schreiber, J. Am. Chem. Soc., 118, 11054 (1996).
mesylate (Step A). The adduct was cyclized as a 1,3-dioxane and further elabo-
rated to an aldehyde intermediate. Reduction to an allylic alcohol by Red-Al
was followed by Sharpless epoxidation. The epoxide was opened by a second
Red-Al reduction. After protecting group manipulation, the aldehyde functional group
was obtained by Swern oxidation. (Steps C-1 to C-7). This aldehyde was coupled