Page 1242 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1242
1218 PhCH 2 O O
PhCH 2 O OCH 2 Ph
1) SmI 2 TBDMSO
CHAPTER 13
Br CH O
Multistep Syntheses O O OPMB 2) Ac 2 O, DMP O 2 CCH 3
TBDMS PMBO OCH 2 Ph
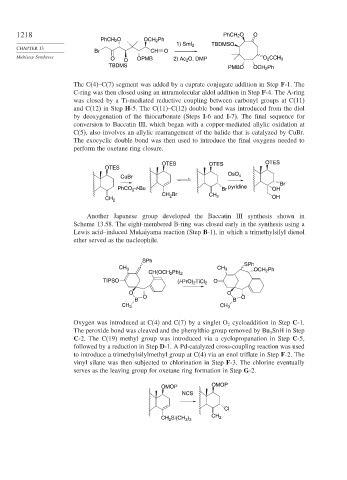
The C(4)–C(7) segment was added by a cuprate conjugate addition in Step F-1. The
C-ring was then closed using an intramolecular aldol addition in Step F-4. The A-ring
was closed by a Ti-mediated reductive coupling between carbonyl groups at C(11)
and C(12) in Step H-5. The C(11)–C(12) double bond was introduced from the diol
by deoxygenation of the thiocarbonate (Steps I-6 and I-7). The final sequence for
conversion to Baccatin III, which began with a copper-mediated allylic oxidation at
C(5), also involves an allylic rearrangement of the halide that is catalyzed by CuBr.
The exocyclic double bond was then used to introduce the final oxygens needed to
perform the oxetane ring closure.
OTES OTES OTES
OTES
OsO
CuBr 4
Br
-t-Bu pyridine
PhCO 3 Br OH
CH Br CH
CH 2 2 2 OH
Another Japanese group developed the Baccatin III synthesis shown in
Scheme 13.58. The eight-membered B-ring was closed early in the synthesis using a
Lewis acid–induced Mukaiyama reaction (Step B-1), in which a trimethylsilyl dienol
ether served as the nucleophile.
SPh
SPh
CH 3 CH 3 OCH 2 Ph
CH(OCH 2 Ph) 2
TIPSO (i-PrO) 2 TiCl 2 O
O O
B O B O
CH 3 CH 3
Oxygen was introduced at C(4) and C(7) by a singlet O cycloaddition in Step C-1.
2
The peroxide bond was cleaved and the phenylthio group removed by Bu SnH in Step
3
C-2. The C(19) methyl group was introduced via a cyclopropanation in Step C-5,
followed by a reduction in Step D-1. A Pd-catalyzed cross-coupling reaction was used
to introduce a trimethylsilylmethyl group at C(4) via an enol triflate in Step F-2. The
vinyl silane was then subjected to chlorination in Step F-3. The chlorine eventually
serves as the leaving group for oxetane ring formation in Step G-2.
OMOP OMOP
NCS
Cl
CH Si(CH ) CH 2
2
3 3