Page 1241 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1241
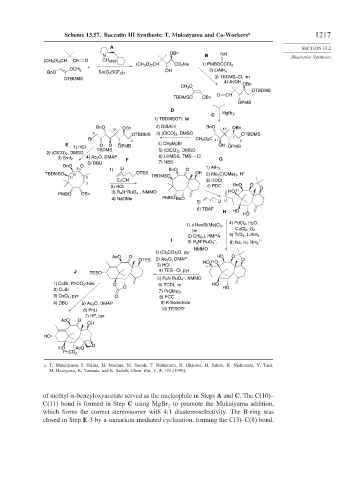
Scheme 13.57. Baccatin III Synthesis: T. Mukaiyama and Co-Workers a 1217
A SECTION 13.2
OBn
N B NH Illustrative Syntheses
(CH 3 O) 2 CH CH O CH 3 NH
+ (CH 3 O) 2 CH CO 2 Me 1) PMBOCCCl 3
OCH 3 OH 2) LiAlH 4
BnO Sn(O 3 SCF 3 ) 2
OTBDMS 3) TBDMS–Cl, Im
4) AcOH OBn
CH 3 O
OTBDMS
O CH
TBDMSO OBn
OPMB
D
C MgBr 2
1) TBDMSOTf, lut
BnO OBn 2) DiBAlH BnO OBn
15 15
8 OTBDMS 3) (ClCO) 2 , DMSO OTBDMS
Br CH 3 O 2 C 11 1
3 3
E O O OPMB 4) CH 3 MgBr OH
1) HCl OPMB
TBDMS 5) (ClCO) 2 , DMSO
2) (ClCO) 2 , DMSO
4) Ac 2 O, DMAP 6) LHMDS, TMS Cl
3) SmI 2 F G
5) DBU 7) NBS
BnO O
9 1) Li BnO O 1) AlH 3
TBDMSO 10 OTES TBDMSO OH 2) Me 2 C(OMe) 2 , H +
11 8
CuCN 3) DDCl
3 BnO O
2) HCl 4) PDC O
–
+
3) R 4 N RuO 4 , NMMO HO 11
PMBO OBn
4) NaOMe PMBO BnO
5) Li 12
6) TBAF
H HO
HO
4) PdCl 2 , H 2 O,
1) c-HexSi(Me)Cl 2 ,
Im CuCl 2 , O 2
2) CH 3 Li, HMPA 5) TiCl 4 , LiAlH 4
I + – 11
3) R 4 N RuO 4 , 6) Na, liq. NH 3
NMMO
1) (Cl 3 CO) 2 O, pyr
AcO O HO O
OTES 2) Ac 2 O, DMAP HO HO O
3) HCl
J TESO 4) TES Cl, pyr
–
5) R 4 N RuO 4 , NMMO
1) CuBr, PhCO 3 -t-Bu O 6) TCDI, im HO
2) CuBr O HO
7) P(OMe) 3
3) OsO 4 , pyr O 8) PCC
4) DBU 5) Ac 2 O, DMAP 9) K-Selectride
6) PhLi 10) TESOTf
7) HF, pyr
AcO O
OH
HO
O
HO AcO
PhCO 2
a. T. Mukaiyama, I. Shiina, H. Iwadare, M. Saitoh, T. Nishimura, N. Ohkawa, H. Sakoh, K. Nishimura, Y. Tani,
M. Hasegawa, K. Yamada, and K. Saitoh, Chem. Eur. J., 5, 121 (1999).
of methyl -benzyloxyacetate served as the nucleophile in Steps A and C. The C(10)–
C(11) bond is formed in Step C using MgBr to promote the Mukaiyama addition,
2
which forms the correct stereoisomer with 4:1 diastereoselectivity. The B-ring was
closed in Step E-3 by a samarium-mediated cyclization, forming the C(3)–C(8) bond.