Page 1240 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1240
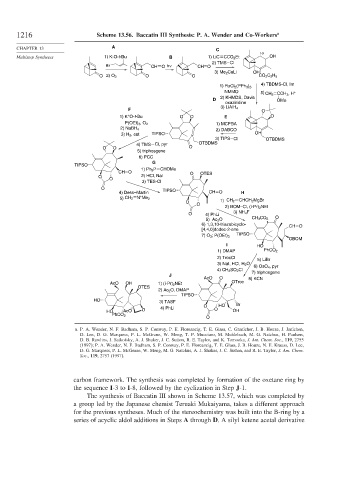
1216 Scheme 13.56. Baccatin III Synthesis: P. A. Wender and Co-Workers a
CHAPTER 13 A C
10
Multistep Syntheses 1) K-O-t-Bu B 1) LiC CCO 2 Et OH
2) TMS Cl
Br CH O hν CH O
3) Me 2 CuLi OH
O 2) O 3 O O CO 2 C 2 H 5
1) RuCl 2 (PPh 3 ) 2 , 4) TBDMS-Cl, Im
NMMO 5) CH 2 CCH 3 , H +
2) KHMDS, Davis
D OMe
oxaziridine
F 3) LiAlH 4
O
+
1) K O-t-Bu O O E O
P(OEt) 3 , O 2 1) MCPBA
2) DABCO
2) NaBH 4
3) H 2 , cat TIPSO OH
3) TIPS Cl OTBDMS
4) TMS Cl, pyr OTBDMS
O O O
5) triphosgene
6) PCC
G
TIPSO
1) Ph 3 P CHOMe
CH O O OTES
O 2) HCl, NaI
O
3) TES-Cl
O TIPSO
4) Dess–Martin CH O H
+
5) CH 2 N Me 2 1) CHCH 2 MgBr
O CH 2
O
2) BOM–Cl, (i-Pr) 2 NEt
O 4) PhLi 3) NH 4 F
5) Ac 2 O CH 3 CO 2 O
6) 1,3,10-triazabicyclo- CH O
[4,4,0]dodec-2-ene
TIPSO
7) O 3 ; P(OEt) 3
OBOM
I HO
1) DMAP PhCO 2
2) TrocCl 5) LiBr
3) NaI, HCl, H 2 O
6) OsO 4 , pyr
4) CH 3 SO 2 Cl
J 7) triphosgene
AcO O 8) KCN
AcO OH 1) (i-Pr) 2 NEt OTroc
OTES
2) Ac 2 O, DMAP
TIPSO
HO
3) TASF
O HO Br
4) PhLi
HO AcO O O OH
PhCO 2
O
a. P. A. Wender, N. F. Badham, S. P. Conway, P. E. Floreancig, T. E. Glass, C. Granicher, J. B. Houze, J. Janichen,
D. Lee, D. G. Marquess, P. L. McGrane, W. Meng, T. P. Mucciaro, M. Muhlebach, M. G. Natchus, H. Paulsen,
D. B. Rawlins, J. Satkofsky, A. J. Shuker, J. C. Sutton, R. E. Taylor, and K. Tomooka, J. Am. Chem. Soc., 119, 2755
(1997); P. A. Wender, N. F. Badham, S. P. Conway, P. E. Floreancig, T. E. Glass, J. B. Houze, N. E. Krauss, D. Lee,
D. G. Marquess, P. L. McGrane, W. Meng, M. G. Natchus, A. J. Shuker, J. C. Sutton, and R. E. Taylor, J. Am. Chem.
Soc., 119, 2757 (1997).
carbon framework. The synthesis was completed by formation of the oxetane ring by
the sequence I-3 to I-8, followed by the cyclization in Step J-1.
The synthesis of Baccatin III shown in Scheme 13.57, which was completed by
a group led by the Japanese chemist Teruaki Mukaiyama, takes a different approach
for the previous syntheses. Much of the stereochemistry was built into the B-ring by a
series of acyclic aldol additions in Steps A through D. A silyl ketene acetal derivative