Page 1235 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1235
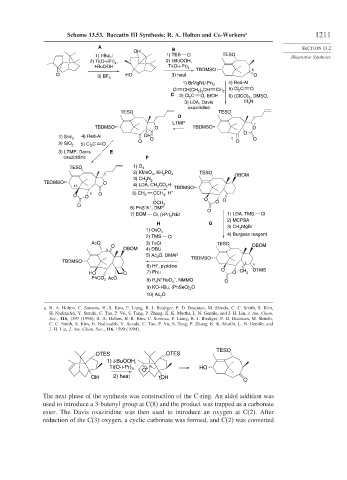
Scheme 13.53. Baccatin III Synthesis: R. A. Holton and Co-Workers a 1211
A SECTION 13.2
OH B
1) t-BuLi 1) TES Cl TESO Illustrative Syntheses
2) Ti(O-i-Pr) , 2) t-BuOOH,
4
t-BuOOH Ti(O-i-Pr) 4
TBDMSO 8
O HO 3) heat O
3) BF 3
1) BrMgN(i-Pr) 2 4) Red-Al
O CH(CH ) CH CH 2 5) Cl C O
2
2 2
C 2) Cl C O, EtOH 6) (ClCO) , DMSO,
2
2
3) LDA, Davis Et 3 N
oxaziridine
TESO TESO
D
LTMP 7
TBDMSO O TBDMSO O
O
4) Red-Al OH
1) SmI 2 O 2 O
O O
5) Cl C O
2) SiO 2
2
3) LTMP, Davis E
oxaziridine F
TESO 1) O 3
TESO
2) KMnO 4 , KH 2 PO 4
OBOM
3) CH N
2
2
TBDMSO O 4) LDA, CH CO H
15 3 2
TBDMSO
2 O 5) CH CCH , H +
O O 2 3
O O
OCH O
O – + 3
6) PhS K , DMF
O
7) BOM Cl, (i-Pr) NEt 1) LDA, TMS Cl
2
2) MCPBA
H G
3) CH 3 MgBr
1) OsO 4
4) Burgess reagent
2) TMS Cl
AcO 3) TsCl TESO
O OBOM
9 OBOM 4) DBU
5) Ac O, DMAP
2 TBDMSO
TBDMSO 4
6) HF, pyridine 5
O CH OTMS
HO O 7) PhLi O 2
PhCO AcO + –
2 8) R N RuO , NMMO O
4 4
O
9) KO-t-Bu, (PhSeO) 2
10) Ac O
2
a. R. A. Holton, C. Somoza, H.-B. Kim, F. Liang, R. J. Biediger, P. D. Boatman, M. Shindo, C. C. Smith, S. Kim,
H. Nadizadeh, Y. Suzuki, C. Tao, P. Vu, S. Tang, P. Zhang, K. K. Murthi, L. N. Gentile, and J. H. Lin, J. Am. Chem.
Soc., 116, 1597 (1994); R. A. Holton, H.-B. Kim, C. Somoza, F. Liang, R. J. Biediger, P. D. Boatman, M. Shindo,
C. C. Smith, S. Kim, H. Nadizadeh, Y. Suzuki, C. Tao, P. Vu, S. Tang, P. Zhang, K. K. Murthi, L. N. Gentile, and
J. H. Liu, J. Am. Chem. Soc., 116, 1599 (1994).
TESO
OTES OTES
1) t-BuOOH,
Ti(O-i-Pr) 4 O HO
OH 2) heat OH
O
The next phase of the synthesis was construction of the C-ring. An aldol addition was
used to introduce a 3-butenyl group at C(8) and the product was trapped as a carbonate
ester. The Davis oxaziridine was then used to introduce an oxygen at C(2). After
reduction of the C(3) oxygen, a cyclic carbonate was formed, and C(2) was converted