Page 1230 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1230
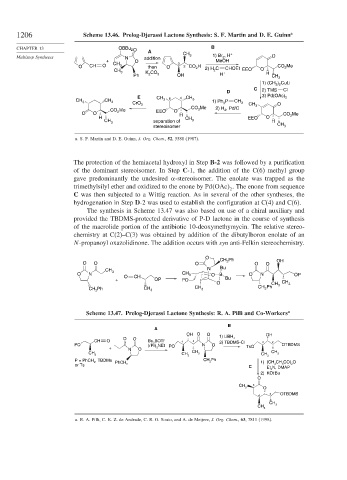
1206 Scheme 13.46. Prelog-Djerassi Lactone Synthesis: S. F. Martin and D. E. Guinn a
CHAPTER 13 OBBu 2O B
A CH
Multistep Syntheses N addition 3 1) Br 2 , H + O
+ O MeOH
O CH O CH 3 then O 3 2 CO H C CHOEt EEO O CO 2 Me
2
CH 3 K CO 2) H 2 + H
Ph 2 3 OH H CH
3
1) (CH 3 ) 2 CuLi
C 2) TMS Cl
D
E CH 4 CH 3 3) Pd(OAc) 2
CH 3 CH 3 3 6 1) Ph P CH
CrO 3 3 3 CH O
CO Me 2) H , Pd/C 3
CO Me EEO O 2 2
O O 2 H CO 2 Me
H EEO O
separation of CH 3 H
CH 3
stereoisomer CH 3
a. S. F. Martin and D. E. Guinn, J. Org. Chem., 52, 5588 (1987).
The protection of the hemiacetal hydroxyl in Step B-2 was followed by a purification
of the dominant stereoisomer. In Step C-1, the addition of the C(6) methyl group
gave predominantly the undesired -stereoisomer. The enolate was trapped as the
trimethylsilyl ether and oxidized to the enone by Pd OAc . The enone from sequence
2
C was then subjected to a Wittig reaction. As in several of the other syntheses, the
hydrogenation in Step D-2 was used to establish the configuration at C(4) and C(6).
The synthesis in Scheme 13.47 was also based on use of a chiral auxiliary and
provided the TBDMS-protected derivative of P-D lactone in the course of synthesis
of the macrolide portion of the antibiotic 10-deoxymethymycin. The relative stereo-
chemistry at C(2)–C(3) was obtained by addition of the dibutylboron enolate of an
N-propanoyl oxazolidinone. The addition occurs with syn anti-Felkin stereochemistry.
O CH Ph
O O O 2 O O OH
CH N Bu
O N 3 CH 3 O B O N OP
O CH Bu
+ OP PO
O CH 3 CH 3
Ph CH CH 3 CH Ph
CH 2 3 2
Scheme 13.47. Prelog-Djerassi Lactone Synthesis: R. A. Pilli and Co-Workers a
B
A
OH O O OH
O O 1) LiBH 4
CH O Bu BOTf 4 2 2) TBDMS-Cl 4 2
2
PO (i Pr) NEt PO N O TsO OTBDMS
+ N O 2
CH 3 CH 3
CH 3 CH
3
CH 3
P = PhCH , TBDMs PhCH CH 2 Ph 1) (CH CH CO) O
or Ts 2 2 C 3 3 2
Et N, DMAP
3
2) KOt Bu
O
6
O
CH 3
2 OTBDMS
4
CH
CH 3
3
a. R. A. Pilli, C. K. Z. de Andrade, C. R. O. Souto, and A. de Meijere, J. Org. Chem., 63, 7811 (1998).