Page 1225 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1225
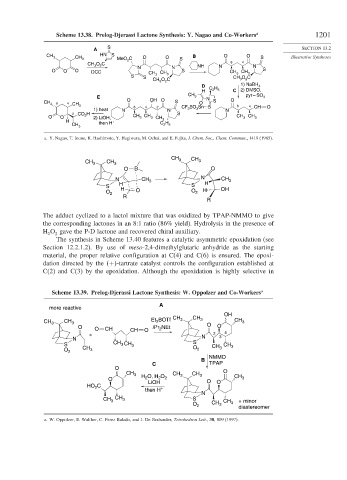
Scheme 13.38. Prelog-Djerassi Lactone Synthesis: Y. Nagao and Co-Workers a 1201
S
A SECTION 13.2
CH 3 CH 3 HN S MeO C O O S B O O S Illustrative Syntheses
CH O C 2 NH N 6 4 N
2
3
O O O DCC N N S CH CH S
3
S S CH 3 CH 3 CH O C 3
CH O C 3 2
3 2
D H 1) NaBH 4
H C 2 5 C 2) DMSO,
CH pyr – SO
E 3 N 3
CH 3 6 4 CH 3 O OH O S O S O
3
1) heat N 6 4 2 N CF 3 SO Sn S N 6 4 CH O
2 CO 2 H S
O O 2) LiOH, CH CH 3 CH CH 3 CH 3
3
H then H + 3 H
CH 3 C 2 5
a. Y. Nagao, T. Inoue, K. Hashimoto, Y. Hagiwara, M. Ochai, and E. Fujita, J. Chem. Soc., Chem. Commun., 1419 (1985).
CH 3 CH
CH 3 CH 3 3
O B O
N CH 3 N CH 3
S H H S H OH
O 2 O O 2 H
R
R
The adduct cyclized to a lactol mixture that was oxidized by TPAP-NMMO to give
the corresponding lactones in an 8:1 ratio (86% yield). Hydrolysis in the presence of
H O gave the P-D lactone and recovered chiral auxiliary.
2 2
The synthesis in Scheme 13.40 features a catalytic asymmetric epoxidation (see
Section 12.2.1.2). By use of meso-2,4-dimethylglutaric anhydride as the starting
material, the proper relative configuration at C(4) and C(6) is ensured. The epoxi-
dation directed by the + -tartrate catalyst controls the configuration established at
C(2) and C(3) by the epoxidation. Although the epoxidation is highly selective in
Scheme 13.39. Prelog-Djerassi Lactone Synthesis: W. Oppolzer and Co-Workers a
A
more reactive
OH
CH CH
CH 3 CH 3 Et 2 BOTf 3 3 CH 3
O O CH CH O i Pr NEt O O
2
+ 2 4
N N 3
3
S CH CH 3 S CH CH 3
CH O 2 3
O 2 3
NMMO
B
C TPAP
O
CH 3 H O, H O CH 3 CH 3 O CH
O 2 2 2 3
LiOH O O
HO C
2
then H +
N
CH 3 CH 3 S CH + minor
O 2 CH 3 3
diastereomer
a. W. Oppolzer, E. Walther, C. Perez Balado, and J. De Brabander, Tetrahedron Lett., 38, 809 (1997).