Page 1226 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1226
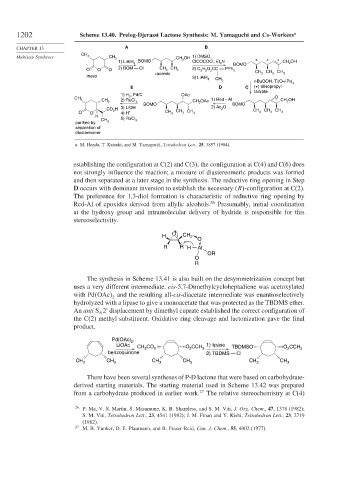
1202 Scheme 13.40. Prelog-Djerassi Lactone Synthesis: M. Yamaguchi and Co-Workers a
CHAPTER 13 A B
Multistep Syntheses CH 3 CH 3 CH 2 OH 1) DMSO,
1) LiAlH BOMO ClCOCOCl, Et 3 N 6 4 2 CH OH
4 2
BOMO
O O O 2) BOM Cl CH 3 CH 3 2) C H O CC PPh 3 CH CH CH
5
2
2
racemic 3 3 3
meso 3) LiAlH
4 CH
3 t-BuOOH, Ti(O-i-Pr)
4
E D C (+)-diisopropyl
tartrate
, Pd/C
1) H 2 OAc O
CH 2) RuCl 3 OAc 1) Red – Al CH 2 OH
CH 3
3 CH 2
BOMO BOMO
2
CO H 3) LiOH 2) Ac O CH CH CH
O O 2 4) H + CH 3 CH 3 CH 3 3 3 3
H
CH 5) RuCl
purified by 3 3
separation of
diastereomer
a. M. Honda, T. Katsuki, and M. Yamaguchi, Tetrahedron Lett., 25, 3857 (1984).
establishing the configuration at C(2) and C(3), the configuration at C(4) and C(6) does
not strongly influence the reaction; a mixture of diastereomeric products was formed
and then separated at a later stage in the synthesis. The reductive ring opening in Step
D occurs with dominant inversion to establish the necessary R -configuration at C(2).
The preference for 1,3-diol formation is characteristic of reductive ring opening by
Red-Al of epoxides derived from allylic alcohols. 26 Presumably, initial coordination
at the hydroxy group and intramolecular delivery of hydride is responsible for this
stereoselectivity.
O
H CH 2
O
R R H Al
OR
O
R
The synthesis in Scheme 13.41 is also built on the desymmetrization concept but
uses a very different intermediate. cis-5,7-Dimethylcycloheptadiene was acetoxylated
with Pd OAc and the resulting all-cis-diacetate intermediate was enantioselectively
2
hydrolyzed with a lipase to give a monoacetate that was protected as the TBDMS ether.
An anti S 2 displacement by dimethyl cuprate established the correct configuration of
N
the C(2) methyl substituent. Oxidative ring cleavage and lactonization gave the final
product.
Pd(OAc) ,
2
LiOAc CH CO 2 O CCH 3 1) lipase TBDMSO O CCH
3
2
benzoquinone 2) TBDMS Cl 2 3
CH 3 CH 3 CH 3 CH 3 CH 3 CH 3
There have been several syntheses of P-D lactone that were based on carbohydrate-
derived starting materials. The starting material used in Scheme 13.42 was prepared
from a carbohydrate produced in earlier work. 27 The relative stereochemistry at C(4)
26 P. Ma, V. S. Martin, S. Masamune, K. B. Sharpless, and S. M. Viti, J. Org. Chem., 47, 1378 (1982);
S. M. Viti, Tetrahedron Lett., 23, 4541 (1982); J. M. Finan and Y. Kishi, Tetrahedron Lett., 23, 2719
(1982).
27
M. B. Yunker, D. E. Plaumann, and B. Fraser-Reid, Can. J. Chem., 55, 4002 (1977).