Page 1223 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1223
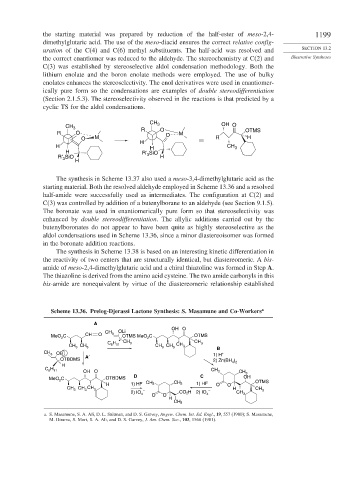
the starting material was prepared by reduction of the half-ester of meso-2,4- 1199
dimethylglutaric acid. The use of the meso-diacid ensures the correct relative config-
uration of the C(4) and C(6) methyl substituents. The half-acid was resolved and SECTION 13.2
the correct enantiomer was reduced to the aldehyde. The stereochemistry at C(2) and Illustrative Syntheses
C(3) was established by stereoselective aldol condensation methodology. Both the
lithium enolate and the boron enolate methods were employed. The use of bulky
enolates enhances the stereoselectivity. The enol derivatives were used in enantiomer-
ically pure form so the condensations are examples of double stereodifferentiation
(Section 2.1.5.3). The stereoselectivity observed in the reactions is that predicted by a
cyclic TS for the aldol condensations.
CH 3 OH
CH 3 O
R O R O O M OTMS
O M R H
H
H H CH 3
H R' SiO
R' SiO 3 H
3
H
The synthesis in Scheme 13.37 also used a meso-3,4-dimethylglutaric acid as the
starting material. Both the resolved aldehyde employed in Scheme 13.36 and a resolved
half-amide were successfully used as intermediates. The configuration at C(2) and
C(3) was controlled by addition of a butenylborane to an aldehyde (see Section 9.1.5).
The boronate was used in enantiomerically pure form so that stereoselectivity was
enhanced by double stereodifferentiation. The allylic additions carried out by the
butenylboronates do not appear to have been quite as highly stereoselective as the
aldol condensations used in Scheme 13.36, since a minor diastereoisomer was formed
in the boronate addition reactions.
The synthesis in Scheme 13.38 is based on an interesting kinetic differentiation in
the reactivity of two centers that are structurally identical, but diastereomeric. A bis-
amide of meso-2,4-dimethylglutaric acid and a chiral thiazoline was formed in Step A.
The thiazoline is derived from the amino acid cysteine. The two amide carbonyls in this
bis-amide are nonequivalent by virtue of the diastereomeric relationship established
Scheme 13.36. Prelog-Djerassi Lactone Synthesis: S. Masamune and Co-Workers a
A
OH O
CH OLi
MeO C CH O 3 OTMS MeO C OTMS
2
2
CH CH
H 3 3
CH CH C 6 11 CH CH CH 3
3 3 3 3
B
CH 3 OB 1) H +
OTBDMS A′ 2) Zn(BH )
H 4 2
C H CH
6 11 OH O 3 CH 3
MeO C OTBDMS D C OH
2 OTMS
H 1) HF CH 3 CH 3 1) HF O O
CH CH
CH 3 3 H CH
3 – – 3
2) IO 4 O O CO 2 H 2) IO 4 CH 3
H
CH 3
a. S. Masamune, S. A. Ali, D. L. Snitman, and D. S. Garvey, Angew. Chem. Int. Ed. Engl., 19, 557 (1980); S. Masamune,
M. Hirama, S. Mori, S. A. Ali, and D. S. Garvey, J. Am. Chem. Soc., 103, 1568 (1981).