Page 1219 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1219
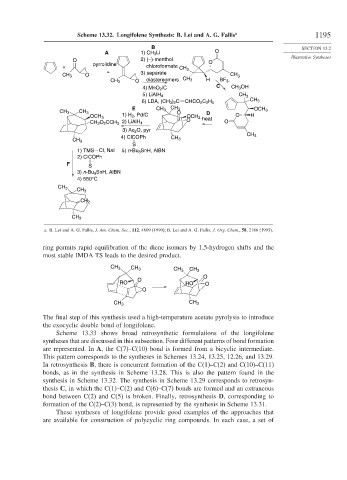
Scheme 13.32. Longifolene Synthesis: B. Lei and A. G. Fallis a 1195
B SECTION 13.2
A 1) CH 3 Li O
Illustrative Syntheses
O 2) (–)-menthol O
+ pyrrolidine chloroformate CH 3
CH 3 O 3) separate CH 3
O diastereomers CH 3 H BF 3 ,
CH 3
4) MnO 2 /C C CH 3 OH
CH
5) LiAIH 4 3
6) LDA, (CH 3 ) 2 C CHCO 2 C 2 H 5 CH 3
E CH 3 CH 3
CH 3 CH 3 O D OCH 3
1) H 2 , Pd/C O H
OCH 3 OCH 3 heat
O O
CH 2 O 2 CCH 3 2) LiAlH 4
3) Ac 2 O, pyr
CH 3
4) ClCOPh
CH 3 CH 3
S
1) TMS Cl, NaI 5) n-Bu 3 SnH, AlBN
2) ClCOPh
F S
3) n-Bu 3 SnH, AlBN
4) 550°C
CH 3
CH 3
CH 2
CH 3
a. B. Lei and A. G. Fallis, J. Am. Chem. Soc., 112, 4609 (1990); B. Lei and A. G. Fallis, J. Org. Chem., 58, 2186 (1993).
ring permits rapid equilibration of the diene isomers by 1,5-hydrogen shifts and the
most stable IMDA TS leads to the desired product.
CH 3 CH 3
CH 3 CH 3
O
O
RO RO O
O
CH 3 CH 3
The final step of this synthesis used a high-temperature acetate pyrolysis to introduce
the exocyclic double bond of longifolene.
Scheme 13.33 shows broad retrosynthetic formulations of the longifolene
syntheses that are discussed in this subsection. Four different patterns of bond formation
are represented. In A, the C(7)–C(10) bond is formed from a bicyclic intermediate.
This pattern corresponds to the syntheses in Schemes 13.24, 13.25, 12.26, and 13.29.
In retrosynthesis B, there is concurrent formation of the C(1)–C(2) and C(10)–C(11)
bonds, as in the synthesis in Scheme 13.28. This is also the pattern found in the
synthesis in Scheme 13.32. The synthesis in Scheme 13.29 corresponds to retrosyn-
thesis C, in which the C(1)–C(2) and C(6)–C(7) bonds are formed and an extraneous
bond between C(2) and C(5) is broken. Finally, retrosynthesis D, corresponding to
formation of the C(2)–C(3) bond, is represented by the synthesis in Scheme 13.31.
These syntheses of longifolene provide good examples of the approaches that
are available for construction of polycyclic ring compounds. In each case, a set of