Page 1214 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1214
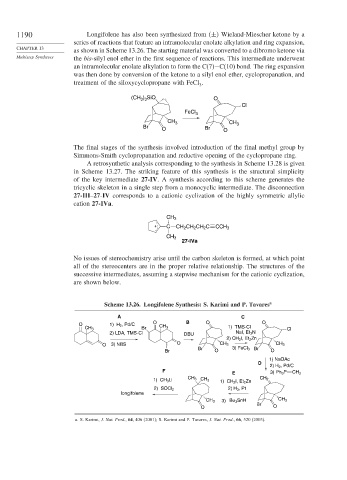
1190 Longifolene has also been synthesized from ± Wieland-Miescher ketone by a
series of reactions that feature an intramolecular enolate alkylation and ring expansion,
CHAPTER 13
as shown in Scheme 13.26. The starting material was converted to a dibromo ketone via
Multistep Syntheses the bis-silyl enol ether in the first sequence of reactions. This intermediate underwent
an intramolecular enolate alkylation to form the C(7)−C(10) bond. The ring expansion
was then done by conversion of the ketone to a silyl enol ether, cyclopropanation, and
treatment of the siloxycyclopropane with FeCl .
3
(CH ) SiO O
3 3
Cl
FeCl 3
CH 3 CH
Br O Br O 3
The final stages of the synthesis involved introduction of the final methyl group by
Simmons-Smith cyclopropanation and reductive opening of the cyclopropane ring.
A retrosynthetic analysis corresponding to the synthesis in Scheme 13.28 is given
in Scheme 13.27. The striking feature of this synthesis is the structural simplicity
of the key intermediate 27-IV. A synthesis according to this scheme generates the
tricyclic skeleton in a single step from a monocyclic intermediate. The disconnection
27-III–27-IV corresponds to a cationic cyclization of the highly symmetric allylic
cation 27-IVa.
CH 3
+ C CH 2 CH 2 CH 2 C CCH 3
CH 3
27-IVa
No issues of stereochemistry arise until the carbon skeleton is formed, at which point
all of the stereocenters are in the proper relative relationship. The structures of the
successive intermediates, assuming a stepwise mechanism for the cationic cyclization,
are shown below.
Scheme 13.26. Longifolene Synthesis: S. Karimi and P. Tavares a
A C
O B O O
O 1) H 2 , Pd/C
CH 3 Br CH 3 1) TMS-Cl Cl
2) LDA, TMS-Cl DBU NaI, Et 3 N
2) CH 2 I, Et 2 Zn
O 3) NBS O CH 3 CH 3
Br 3) FeCl 3 Br
Br O O
1) NaOAc
D
2) H 2 , Pd/C
F CH
E 3) Ph 3 P 2
1) CH 3 Li CH 3 CH 3 1) CH 2 I, Et 2 Zn CH 2
2) SOCl 2 2) H 3 , Pt
longifolene
3) Bu 3 SnH CH 3
CH 3
Br
O O
a. S. Karimi, J. Nat. Prod., 64, 406 (2001); S. Karimi and P. Tavares, J. Nat. Prod., 66, 520 (2003).