Page 1211 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1211
but they are not independent of one another because the geometry of the ring system 1187
requires that they have a specific relative relationship. That does not mean stereo-
chemistry can be ignored, however, since the formation of the various rings will fail SECTION 13.2
if the reactants do not have the proper stereochemistry. Illustrative Syntheses
13 14
CH 3 6 CH 3
7 5
15
10 CH
9 11 2 4
8 1 2 3
CH 3
12
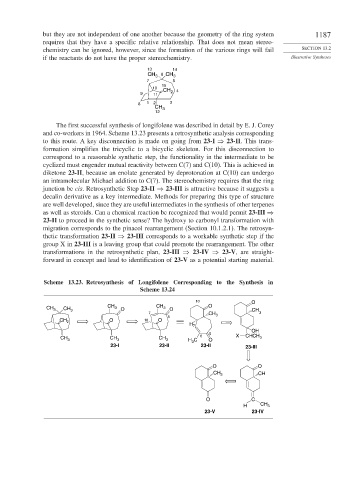
The first successful synthesis of longifolene was described in detail by E. J. Corey
and co-workers in 1964. Scheme 13.23 presents a retrosynthetic analysis corresponding
to this route. A key disconnection is made on going from 23-I ⇒ 23-II. This trans-
formation simplifies the tricyclic to a bicyclic skeleton. For this disconnection to
correspond to a reasonable synthetic step, the functionality in the intermediate to be
cyclized must engender mutual reactivity between C(7) and C(10). This is achieved in
diketone 23-II, because an enolate generated by deprotonation at C(10) can undergo
an intramolecular Michael addition to C(7). The stereochemistry requires that the ring
junction be cis. Retrosynthetic Step 23-II ⇒ 23-III is attractive because it suggests a
decalin derivative as a key intermediate. Methods for preparing this type of structure
are well developed, since they are useful intermediates in the synthesis of other terpenes
as well as steroids. Can a chemical reaction be recognized that would permit 23-III ⇒
23-II to proceed in the synthetic sense? The hydroxy to carbonyl transformation with
migration corresponds to the pinacol rearrangement (Section 10.1.2.1). The retrosyn-
thetic transformation 23-II ⇒ 23-III corresponds to a workable synthetic step if the
group X in 23-III is a leaving group that could promote the rearrangement. The other
transformations in the retrosynthetic plan, 23-III ⇒ 23-IV ⇒ 23-V, are straight-
forward in concept and lead to identification of 23-V as a potential starting material.
Scheme 13.23. Retrosynthesis of Longifolene Corresponding to the Synthesis in
Scheme 13.24
10 O
CH 3 CH 3 CH 3 O CH 3 O O CH
7 CH 3 3
5
CH 2 O 10 O
H 7
OH
5
CH 3 CH 3 CH 3 H C 6 O X CHCH 3
3
23-I 23-II 23-II 23-III
O O
CH
CH 3
O C
H CH 3
23-V 23-IV