Page 1216 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1216
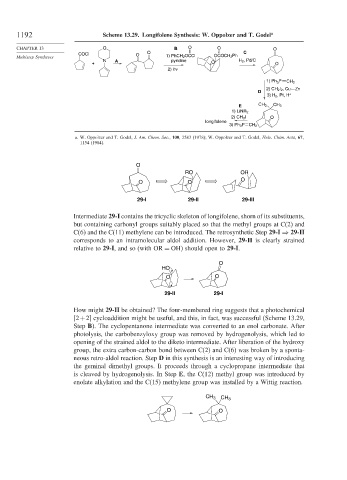
1192 Scheme 13.29. Longifolene Synthesis: W. Oppolzer and T. Godel a
CHAPTER 13 O B O O O
COCl O O C
Multistep Syntheses 1) PhCH 2 OCCl OCOCH 2 Ph
N A pyridine H 2 , Pd/C
+ O O
2) h ν
P
1) Ph 3 CH 2
2) CH 2 I 2 , Cu Zn
D
3) H 2 , Pt, H +
E CH 3 CH 3
1) LiNR 2
2) CH 3 I O
longifolene
3) Ph 3 P CH 2
a. W. Oppolzer and T. Godel, J. Am. Chem. Soc., 100, 2583 (1978); W. Oppolzer and T. Godel, Helv. Chim. Acta, 67,
1154 (1984).
O
RO OR
O
O O
29-I 29-II 29-III
Intermediate 29-I contains the tricyclic skeleton of longifolene, shorn of its substituents,
but containing carbonyl groups suitably placed so that the methyl groups at C(2) and
C(6) and the C(11) methylene can be introduced. The retrosynthetic Step 29-I ⇒ 29-II
corresponds to an intramolecular aldol addition. However, 29-II is clearly strained
relative to 29-I, and so (with OR = OH) should open to 29-I.
O
HO
O O
29-II 29-I
How might 29-II be obtained? The four-membered ring suggests that a photochemical
2+2 cycloaddition might be useful, and this, in fact, was successful (Scheme 13.29,
Step B). The cyclopentanone intermediate was converted to an enol carbonate. After
photolysis, the carbobenzyloxy group was removed by hydrogenolysis, which led to
opening of the strained aldol to the diketo intermediate. After liberation of the hydroxy
group, the extra carbon-carbon bond between C(2) and C(6) was broken by a sponta-
neous retro-aldol reaction. Step D in this synthesis is an interesting way of introducing
the geminal dimethyl groups. It proceeds through a cyclopropane intermediate that
is cleaved by hydrogenolysis. In Step E, the C(12) methyl group was introduced by
enolate alkylation and the C(15) methylene group was installed by a Wittig reaction.
CH 3
CH 3
O O