Page 1217 - Advanced Organic Chemistry Part B - Reactions & Synthesis
P. 1217
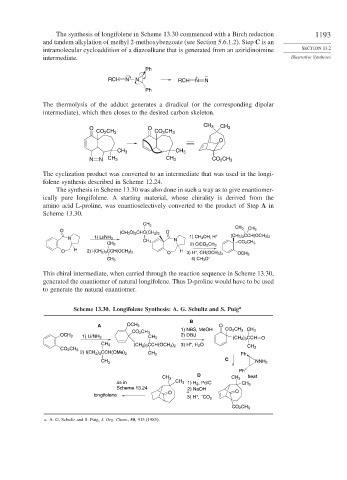
The synthesis of longifolene in Scheme 13.30 commenced with a Birch reduction 1193
and tandem alkylation of methyl 2-methoxybenzoate (see Section 5.6.1.2). Step C is an
intramolecular cycloaddition of a diazoalkane that is generated from an aziridinoimine SECTION 13.2
intermediate. Illustrative Syntheses
Ph
+ –
RCH N N RCH N N
Ph
The thermolysis of the adduct generates a diradical (or the corresponding dipolar
intermediate), which then closes to the desired carbon skeleton.
O O CH 3 CH 3
CO 2 CH 3 CO 2 CH 3
O
CH 3 CH 3
N N CH 3 CH 3 CO 2 CH 3
The cyclization product was converted to an intermediate that was used in the longi-
folene synthesis described in Scheme 12.24.
The synthesis in Scheme 13.30 was also done in such a way as to give enantiomer-
ically pure longifolene. A starting material, whose chirality is derived from the
amino acid L-proline, was enantioselectively converted to the product of Step A in
Scheme 13.30.
CH
3
CH 3
O O CH 3
(CH 3 O) 2 CHC(CH 2 ) 3
1) CH 3 OH, H + (CH 2 ) 3 CCH(OCH 3 ) 2
N 1) Li/NH 3 N
CH 3
CH 3 2) ClCO 2 CH 3 CO 2 CH 3
O H 2) I(CH 2 ) 3 CCH(OCH 3 ) 2 O H 3) H , CH(OCH 3 ) 3 OCH 3
+
4) CH 3 O –
CH 3
This chiral intermediate, when carried through the reaction sequence in Scheme 13.30,
generated the enantiomer of natural longifolene. Thus D-proline would have to be used
to generate the natural enantiomer.
Scheme 13.30. Longifolene Synthesis: A. G. Schultz and S. Puig a
B
A OCH 3 O
1) NBS, MeOH CO 2 CH 3 CH 3
CO 2 CH 3
OCH 3 2) DBU
1) Li/NH 3 CH 3 (CH 2 ) 3 CCH O
+
CH 3 (CH 2 ) 3 CCH(OCH 3 ) 2 3) H , H 2 O
CH 3
CO 2 CH 3
2) I(CH ) CCH(OMe) 2 CH 3 Ph
2 3
CH 3 C NNH 2
Ph
D heat
CH 3 CH 3
as in CH 3 1) H 2 , Pd/C CH 3
Scheme 13.24 2) NaOH
O O
longifolene + –
3) H , CO 2
CO 2 CH 3
a. A. G. Schultz and S. Puig, J. Org. Chem., 50, 915 (1985).